Monday, Sept 30 2024
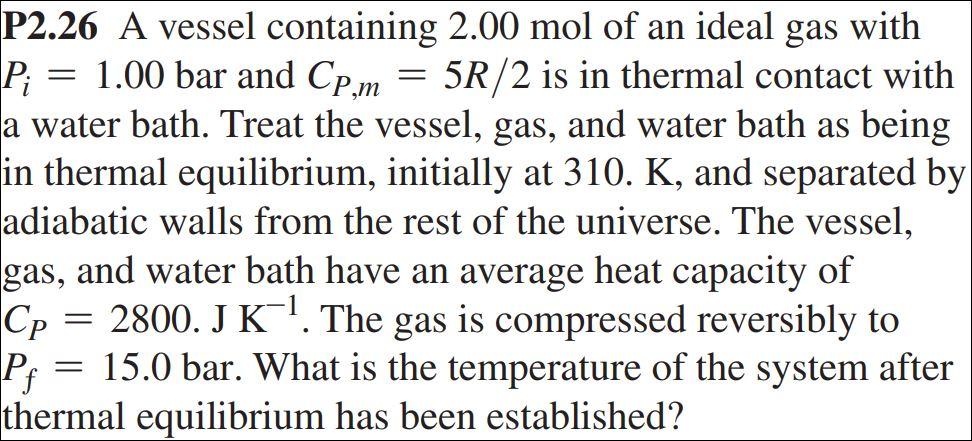
Solved P2.26 A vessel containing 2.00 mol of an ideal gas
By A Mystery Man Writer
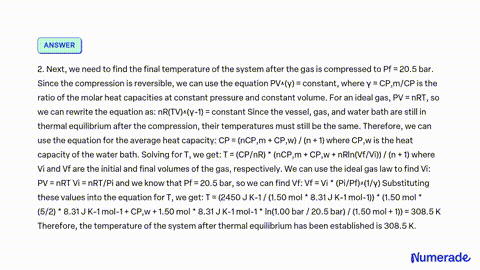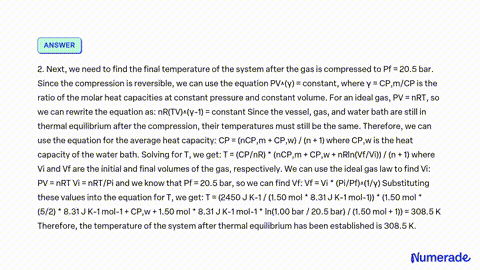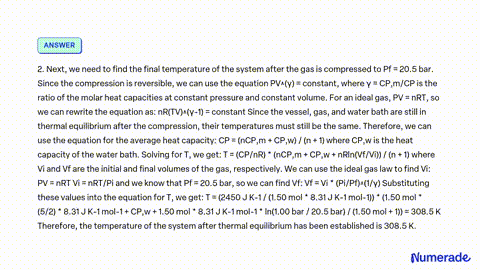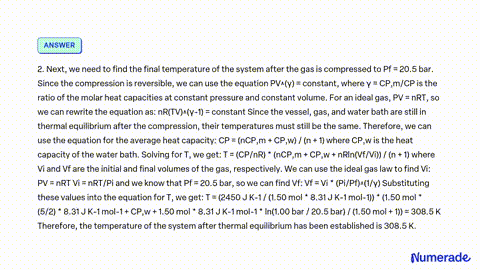
SOLVED: A vessel containing 2.00 mol of an ideal gas with P1 1.00 bar and Cp mR/2 is in thermal contact with a water bath. Treat the vessel, gas, and water bath


A gaseous mixture enclosed in a vessel of volume V consists of one mole of a gas A with γ=5 / 3 a

An ideal gas in thermally insulated vessel at internal (pressure)=P_(1), (volume)=V_(1) and abso
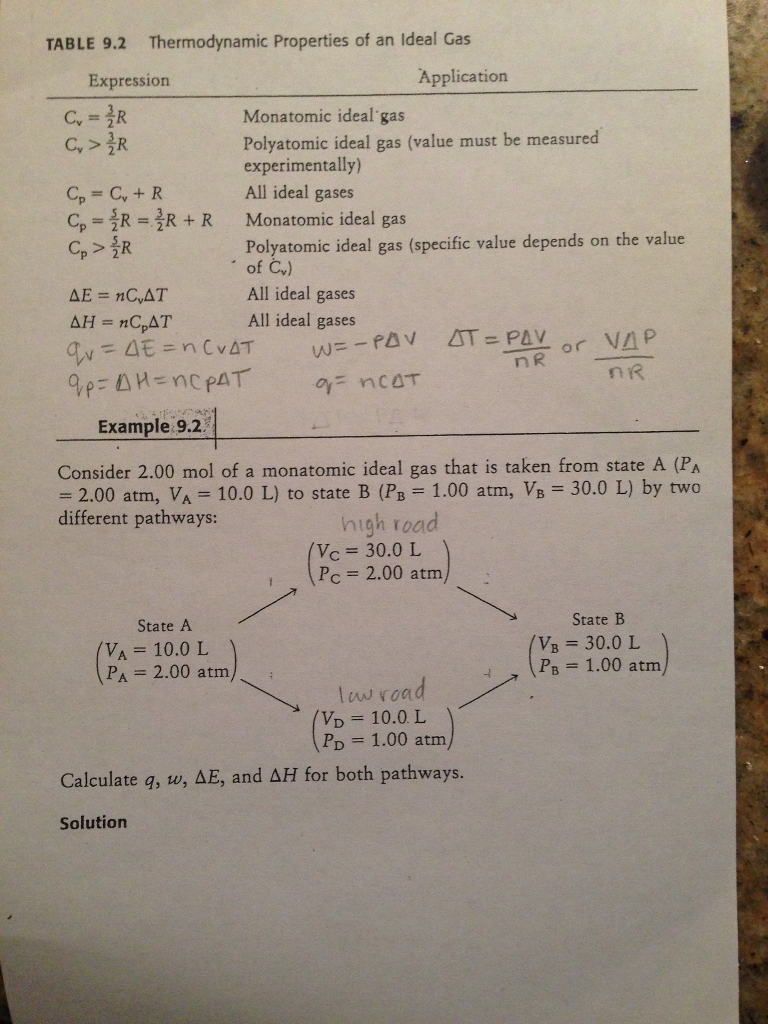
Solved Consider 2.00 mol of a monatomic ideal gas that is

SOLVED: A vessel containing 2.00 mol of an ideal gas with P1 1.00
What pressure is exerted by the mixture of 2.0g of hydrogen and 8.0 g of nitrogen at 273k in a 10l vessel? - Quora

Heat 3

Consider a sample containing 2.00 moles of a monatomic ideal

JEE - MODULE 2 - CHEM - +1 NM Physical Chemistry - 2, PDF
Related searches
- Lipoelastic compression bra PI ideal –

- The Number Pi (π) - 500,000 Decimals: Ideal present or gimmick for nerds, number freaks, pi lovers, math, physics, chemistry, computer science, and science students : Hein, Lima: : Books

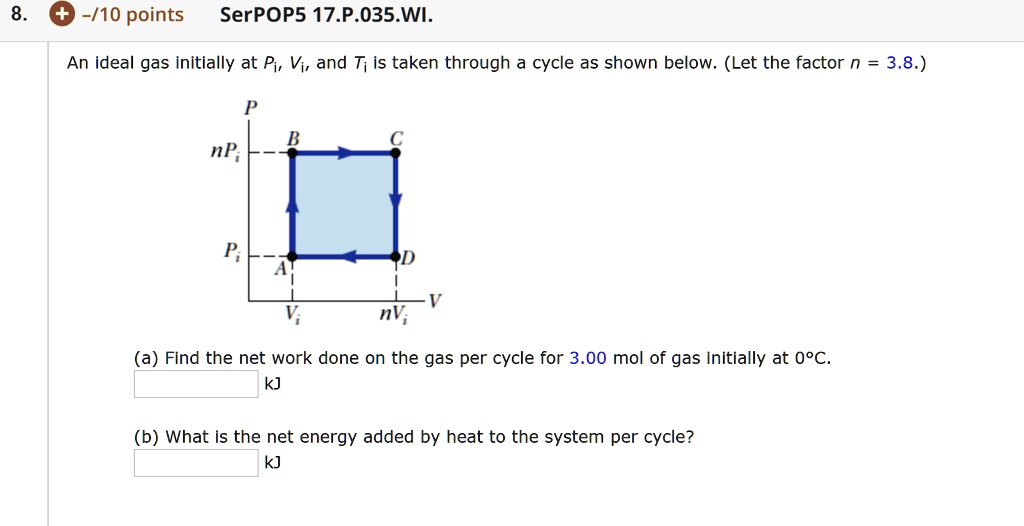
- SOLVED: An ideal gas initially at Pi, Vi, and Ti is taken through a cycle as shown below. (Let the factor n = 3.8.) (a) Find the net work done on the
- Lipoelastic PI Relax - Pink

- Бюстгальтер ортопедический PI-iV

©2016-2024, changhanna.com, Inc. or its affiliates