Sacituzumab Earns Regular FDA Approval for TNBC - NCI

By A Mystery Man Writer
Sacituzumab govitecan (Trodelvy) now has regular FDA approval for people with locally advanced or metastatic triple-negative breast cancer (TNBC), including those with brain metastases. The update follows last year’s accelerated approval of the drug for people with TNBC.

Targeting Triple-negative Breast Cancer

Mission Mountain Wilderness

A Comprehensive Immunologic Portrait of Triple-Negative Breast Cancer - ScienceDirect

Therapeutic efficacy of IMMU-132 with different DARs. NCI-N87

Mission Mountain Wilderness

Recent advances in targeted strategies for triple-negative breast

Sacituzumab govitecan, a Trop-2-directed antibody-drug conjugate
Frontiers Immunotherapy in triple-negative breast cancer: Insights into tumor immune landscape and therapeutic opportunities
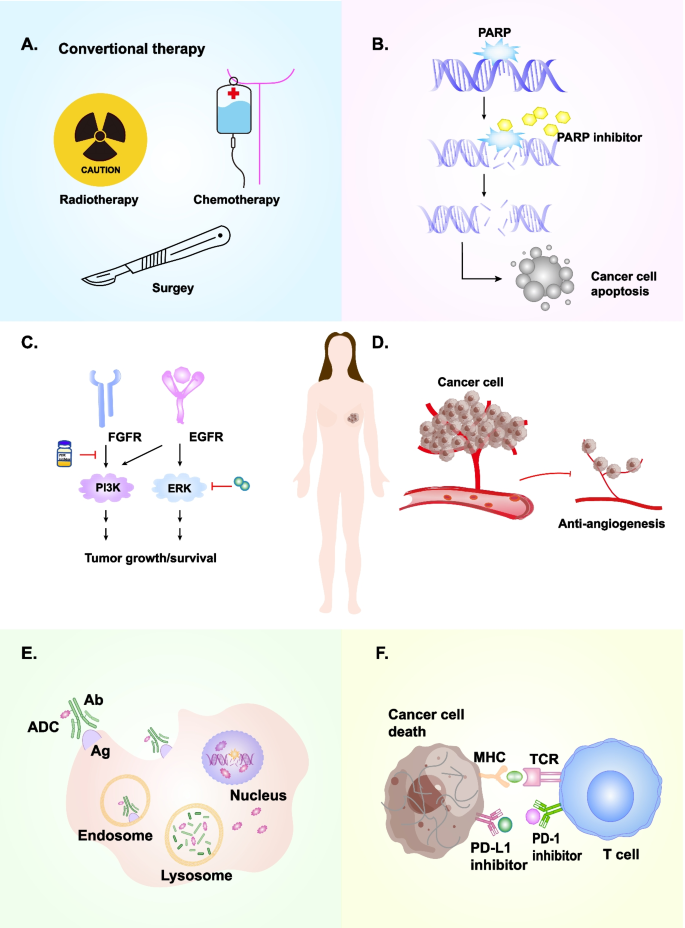
Recent advances in targeted strategies for triple-negative breast









