200 g of a sample of limestone liberates 66 g of CO2 on heating. The percentage purity of CaCO3 in the limestone is Options:a 95
By A Mystery Man Writer
200 g of a sample of limestone liberates 66 g of CO2 on heating. The percentage purity of CaCO3 in the limestone is Options:a 95
200 g of a sample of limestone liberates 66 g of CO2 on heating- The percentage purity of CaCO3 in the limestone is Options-a- 95

200 g of a sample of limestone liberates 66 g of CO2 on heating. The percentage purity of CaCO3 in the limestone is Options:a 95
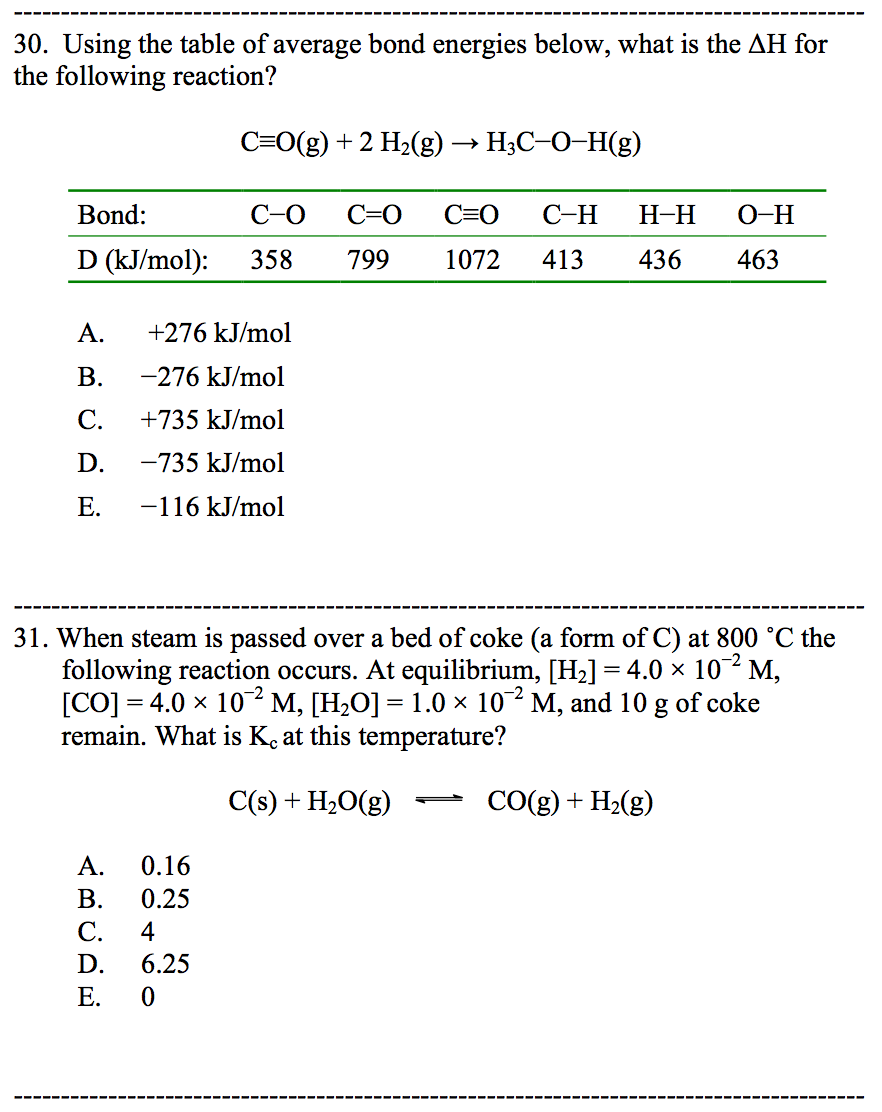
Solved Please help me solve the following questions below
What quantity of commercial limestone containing 80% CaCO3 on heating will give 5.6 kg of CaO? - Quora

⏩SOLVED:A sample of limestone (containing calcium carbonate, CaCO3 )…
200g of a sample of limestone liberates 66 g of co2 on heating. The

Decarbonization
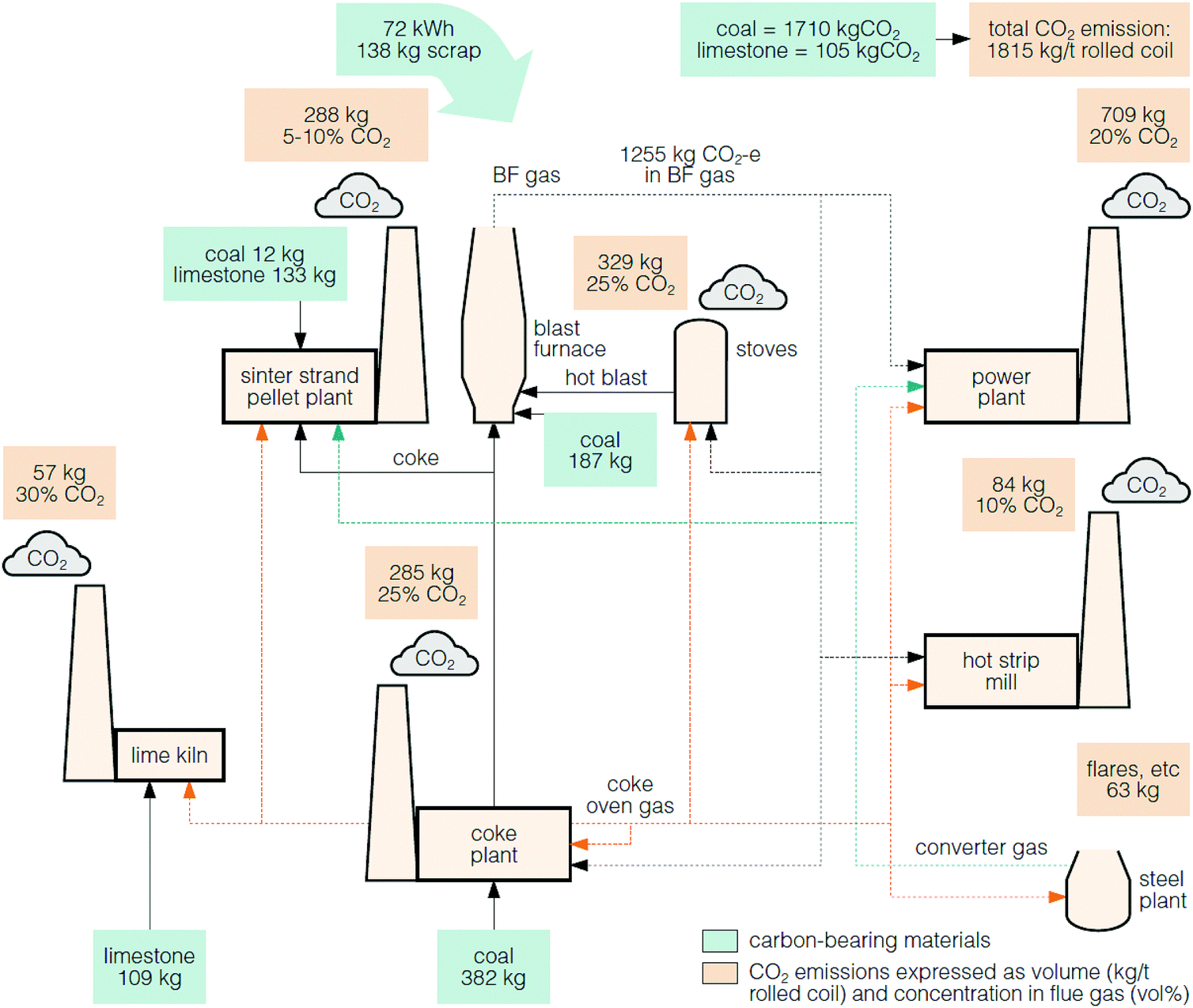
Carbon capture and storage (CCS): the way forward - Energy & Environmental Science (RSC Publishing) DOI:10.1039/C7EE02342A

Solved Analysis of a limestone sample showed that it is

Unical Science 2o22

Wu unswer the questions given below it: 150 ml of N HCI is required to react completely with 1.0 g of a sample of limestone. Calculate the percentage purity of CaCO3. (A)
- How Shein's influencer trip to a Chinese factory backfired

- How to Doll Dress for 18 dolls (American Girl, Our Generation) • Sami Doll Tutorials

- How to Achieve the Appearance of a Butt-Lift without Surgery

- Washable Silk Sleep Eye Mask – ThirdLove

- LEG-R {On Another Page} Black Checkered Leggings EXTENDED PLUS





