Does atomic size increase down a group?

By A Mystery Man Writer
Does atomic size increase down a group?
Does atomic size increase down a group

Periodic Properties and Trends Atomic Radii Size Increases going down a group.Size Increases going down a group. Because electrons are added further. - ppt download

Atomic & Ionic Radius Trend, Definition, Differences & Chart - Lesson
Atomic and ionic radii (video)

Question Video: Identifying Why the Atomic Radius Increases upon Descending a Group on the Periodic Table

define atomic size, give its unit of measure in the modern periodic table what trend is observed in atomic

ANSWERED] Going down a group in the periodic table, atomic size - Kunduz
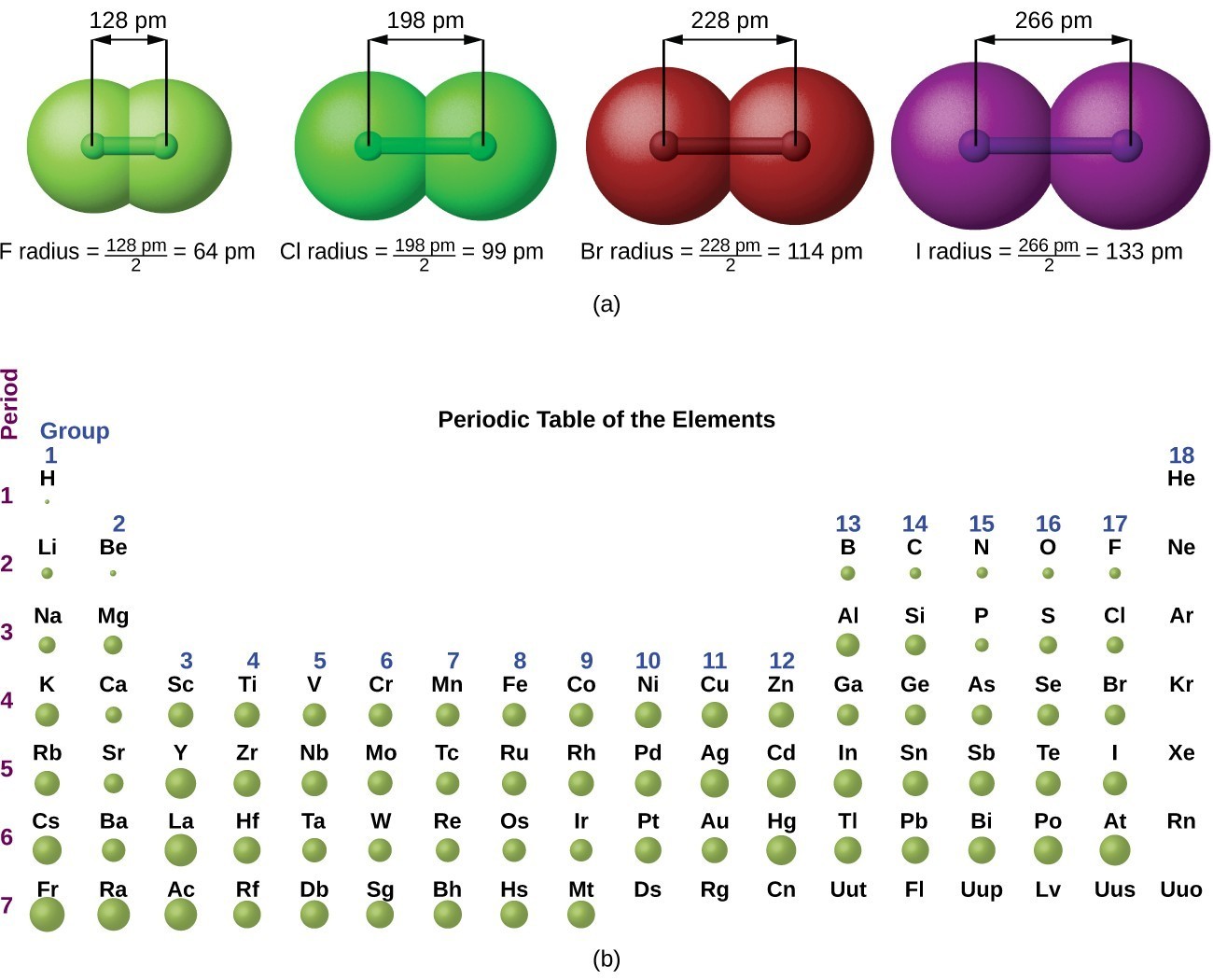
Periodic Variations in Element Properties

SOLVED: Why does atomic size tend to decrease with increasing

SOLUTION: Atomic sizes, Atomic radii, covalent radii, ionic radii

6answer Module Sk015 Chapter 3 1 .pdf - Practice Module Chapter 3

Why does the atomic size increases down a group? - Quora
What are the periodic trends for atomic radii, ionization energy, and electron affinity?
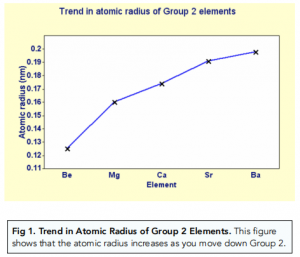
Group 2, The Alkaline Earth Metals (A-Level Chemistry) - Study Mind

1. Atomic radius increases down the group. Why?
- How do Air Force 1 07 fit? Is it tts or half size down? Im TTS on size 9 (Jordans mainly) : r/Sneakers
- Best Duvet And Pillow Sizes Including California King!
- Find Your Size, Roller Skate Size Guide

- Rab Down Jacket Warmth Guide - Ultralight Outdoor Gear

- Always Use “Buttons” for Size Selection (28% of Desktop Sites Don't) – Articles – Baymard Institute