How to Calculate Normality of a Solution
:max_bytes(150000):strip_icc()/how-to-calculate-normality-609580final2-0d5efa5a961f4fa0a7efc780921faee1.png)
By A Mystery Man Writer
The normality of a solution is the gram equivalent weight of a solute per liter of solution. Here are examples of how to calculate the normality.

How to Calculate Normality of a Solution
Calculate the normality of a solution containing 62.3 g of hydrated copper sulphate in 500ml of solution (Cu= 63, S= 32, O=16, H=1)

Finding Normality
:max_bytes(150000):strip_icc()/606823-calculate-molarity-of-a-solution-FINAL-5b7d7e15c9e77c0050355d4e.png)
Learn How to Calculate Molarity of a Solution
:max_bytes(150000):strip_icc()/GettyImages-5368397411-5b428b2ec9e77c0037ee6ea4.jpg)
How to Calculate Normality of a Solution
What is the molarity of 0.4 normality of H2SO4 solution? - Quora

How to calculate the normality of liquids - Quora
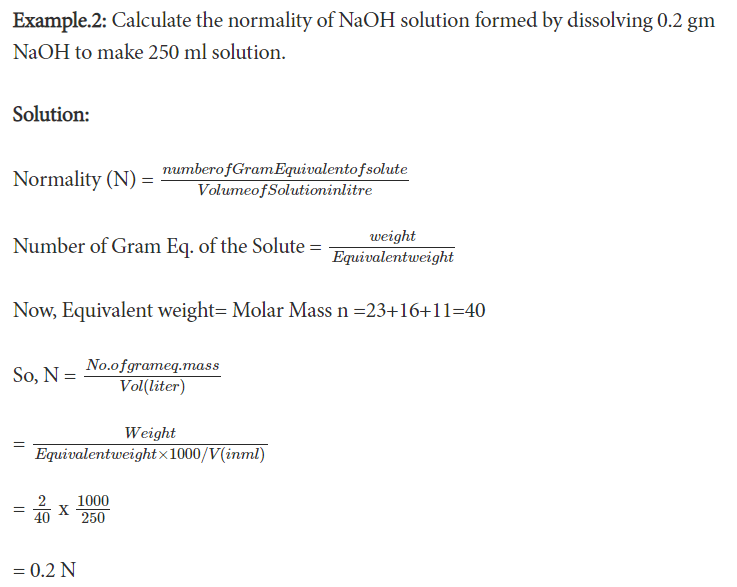
Normality of a solution - Homework Help - Science Forums

11) 1000 (2) TUU 21. Normality of 10% (wV) H,SO, solution is nearly (1) 0.1 (2) 0.2 (3) 0.5 (4) 2

How To Calculate Normality & Equivalent Weight For Acid Base Reactions In Chemistry
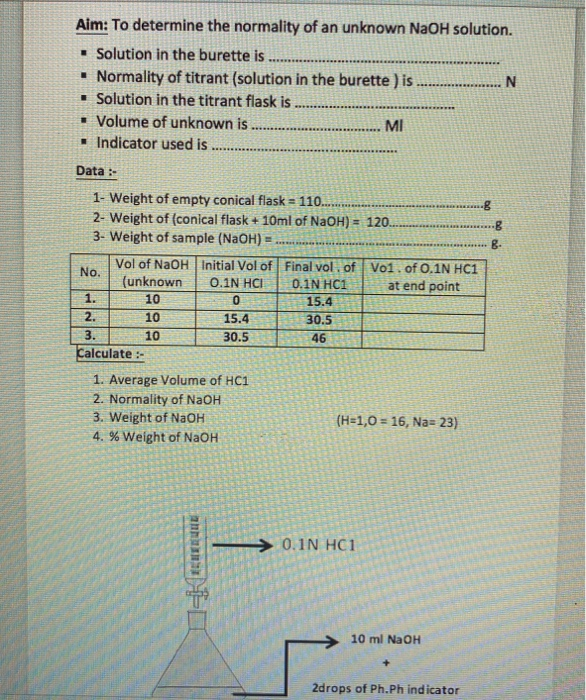
Solved Aim: To determine the normality of an unknown NaOH
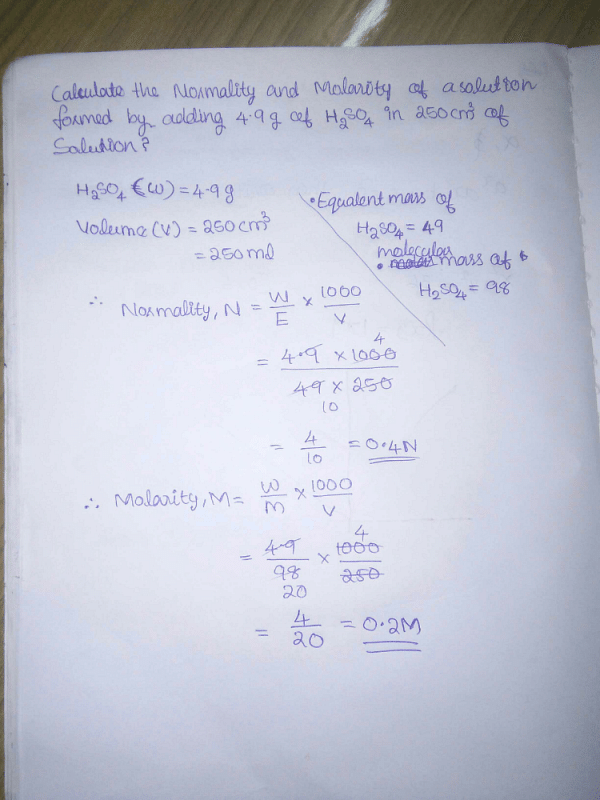
Calculate the Normality and Molarity of a solution formed by adding 4.9g of H2SO4 in 250cm^3 of solution? - EduRev Class 12 Question

What is the normality of solution which contains 10.6 g of Na_2CO_3 in 1250 ml solution ?4/252/258/250.08

Normality,Molality,Molarity,Mole fraction,Formality

NormalityHow to calculate normality of a solution
- Fahrenheit to Celsius Conversion - Edit, Fill, Sign Online
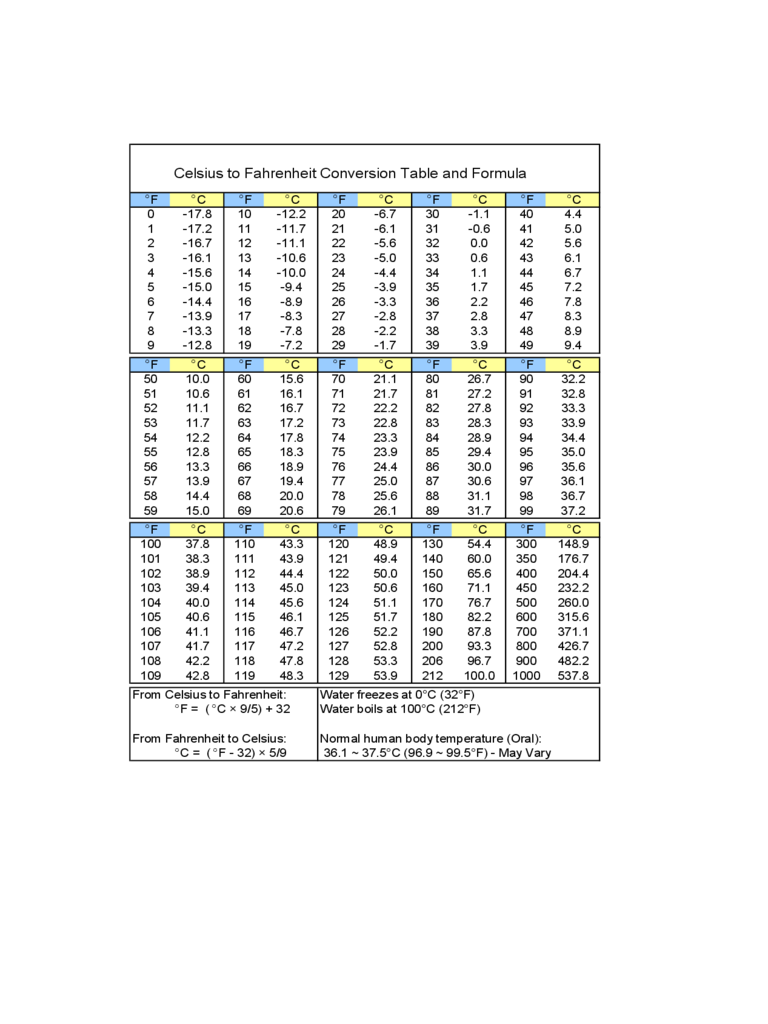
- SOLUTION: Practice problems celsius fahrenheit kevin rankine conversion formula - Studypool

- Solved Normal body temperature varies by time of day. A
- AOGENSI Infrared Non-Contact Digital Forehead Body IR Thermometer Baby Adult

- Snowboard Boot Sizes Conversion Charts

- Our Full Coverage Back Support Posture Bra is designed with back support bands to provide additional shoulder support while gently aiding…
- CABLE KNIT JOGGER – Monanella

- I was told my sports bra gym outfit was 'inappropriate' - but you

- Playtex Secrets Bra: Undercover Slimming Shaping Full-Figure Bra 4S83
- YWDJ Nursing Bras Ladies Comfortable Breathable No Steel Ring Lace



