Ideal gas law, Definition, Formula, & Facts

By A Mystery Man Writer
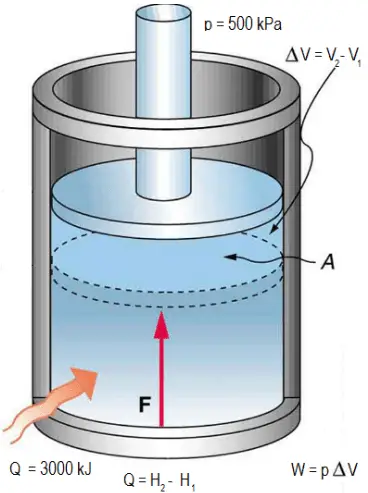
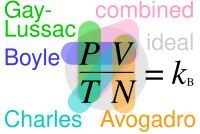
Ideal gas law, relation between the pressure P, volume V, and temperature T of a gas in the limit of low pressures and high temperatures, such that the molecules of the gas move almost independently of each other. In such a case, all gases obey an equation of state known as the ideal gas law: PV =

The Gas Laws - Statements, Formulae, Solved Problems
Ideal gas - Wikipedia

Charles's Law — Overview & Formula - Expii

Lesson Explainer: Boyle's Law

Van der Waals Equation, Definition & Examples - Lesson
:max_bytes(150000):strip_icc()/GettyImages-1044456654-e456c93eeeaf46fe84cbeb0f95814fb6.jpg)
What Is the Ideal Gas Law Definition and Equation?

Avogadro's Law — Overview & Formula - Expii
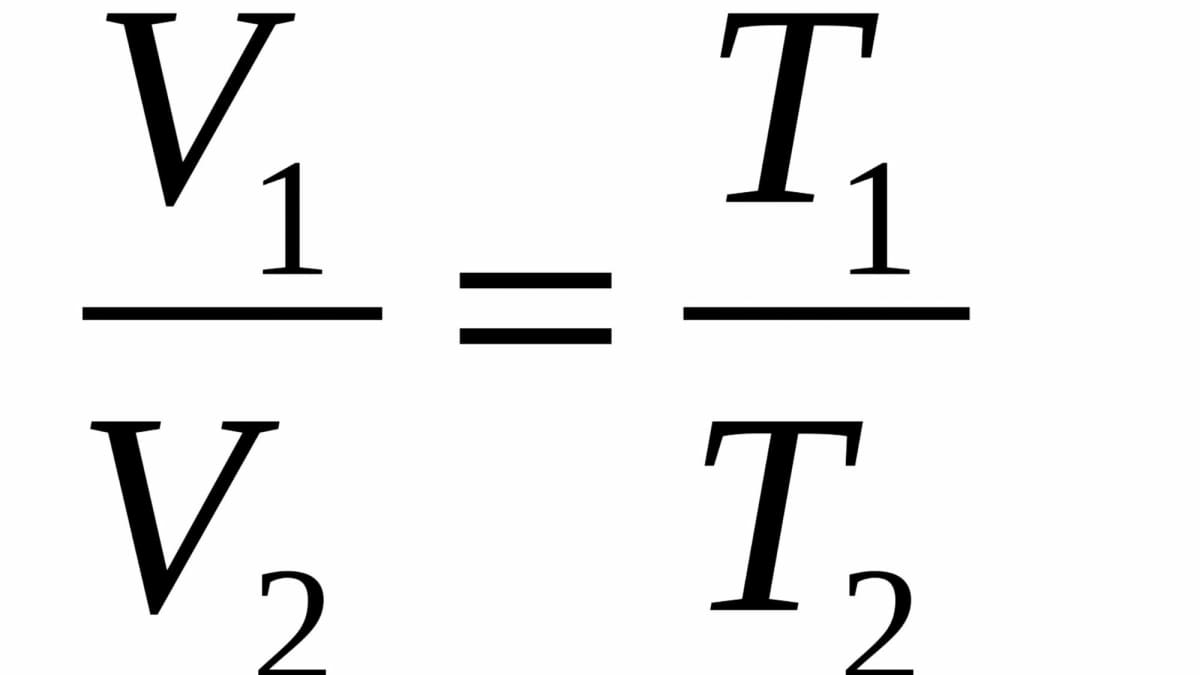
Ideal Gas Law: Volume & Temperature Relationship - Owlcation

Gay-Lussac's Law: Statement, Formula, Explanation, Example & FAQs

Gay-Lussac's Gas Law, Equation and Examples - Lesson
- njshnmn Women's Hiking Pants Fashion Stretch Leggings Sweatpants, Khaki, L

- POLO RALPH LAUREN Big Pony Mesh Polo Shirt Men's Long Sleeve GRAY 3XB NWT

- We Taste Tested Nearly Every Single Grocery Item You Love — Here's What's The Best

- Pilates en la Pared para Mujeres: ¡Reto de 28 Días para una Transformación Extraordinaria! Una Guía Completa con Ejercicios Graduales, Tablas de Entrenamiento y Consejos para una Trayectoria Dirigida

- Female idols who slayed underboob trends
_female-idols-who-slayed-underboob-trends-124-jk39s-pure-soul-124-shorts-kpop-preview-hqdefault.jpg)