Quantum Numbers for Atoms - Chemistry LibreTexts
By A Mystery Man Writer
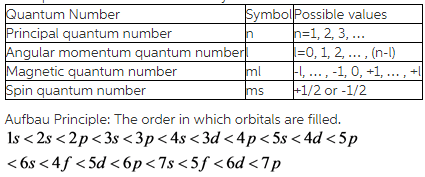
A total of four quantum numbers are used to describe completely the movement and trajectories of each electron within an atom. The combination of all quantum numbers of all electrons in an atom is …
A total of four quantum numbers are used to describe completely the movement and trajectories of each electron within an atom. The combination of all quantum numbers of all electrons in an atom is described by a wave function that complies with the Schrödinger equation. Each electron in an atom has a unique set of quantum numbers; according to the Pauli Exclusion Principle, no two electrons can share the same combination of four quantum numbers.
13.1: Quantum Numbers of Multielectron Atoms - Chemistry LibreTexts
Question #51981
2.2.2: Quantum Numbers and Atomic Wave Functions - Chemistry
Quantum Numbers For Atoms Chemistry LibreTexts, 43% OFF

9.3 - Molecular Orbital Theory - Chemistry LibreTexts

cdn1./wp-content/uploads/2018/07/Quantum

GC1 - Q2 - Week 1, PDF, Atomic Orbital

Las shs gen.chem-melc_1_q2_week-1

Impressions: Robinson's Brutus Awards For 2015, Part, 42% OFF
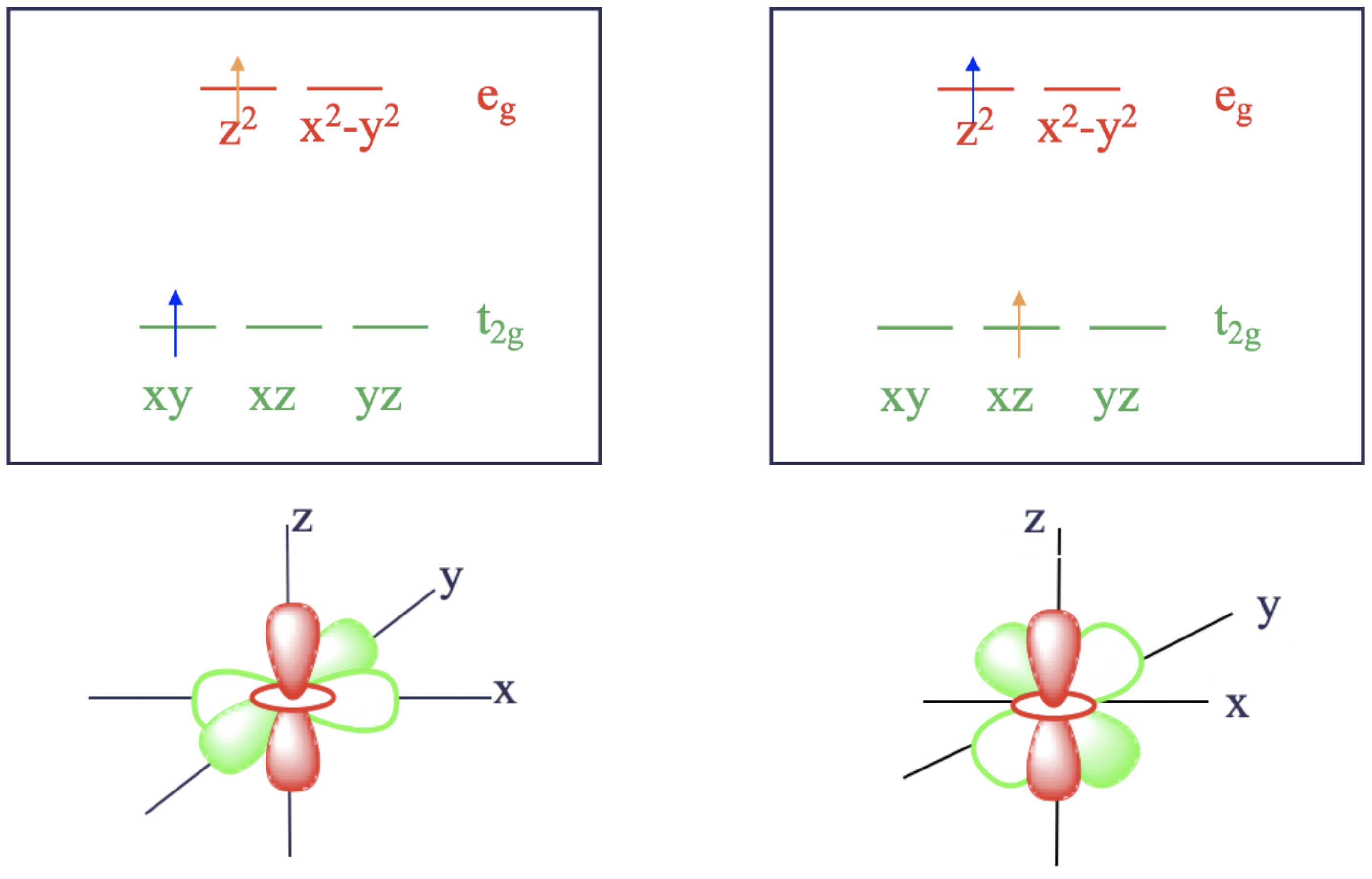
Section 8.2: Quantum Numbers of Multielectron Atoms - Chemistry

Chemical bonding, Definition, Types, & Examples
- Speedo Women's Edge Team Warm Up Pants

- HOLLISTER HERITAGE ICON LOGO STRETCH POLO SHIRT BLACK MENS SIZE

- 4 Pcs Fishnet High Waisted Shorts Mesh Short Leggings See Through Leggings Black Fishnet Tights Cover up Shorts for Women (Floral) : : Clothing, Shoes & Accessories

- Blush Bridesmaids Saree

- Full coverage minimizer bra for Sagging Breast or Heavy Breast #kamison.in @kamison bra