Show that the van der Waals equation leads to values of Z <

By A Mystery Man Writer
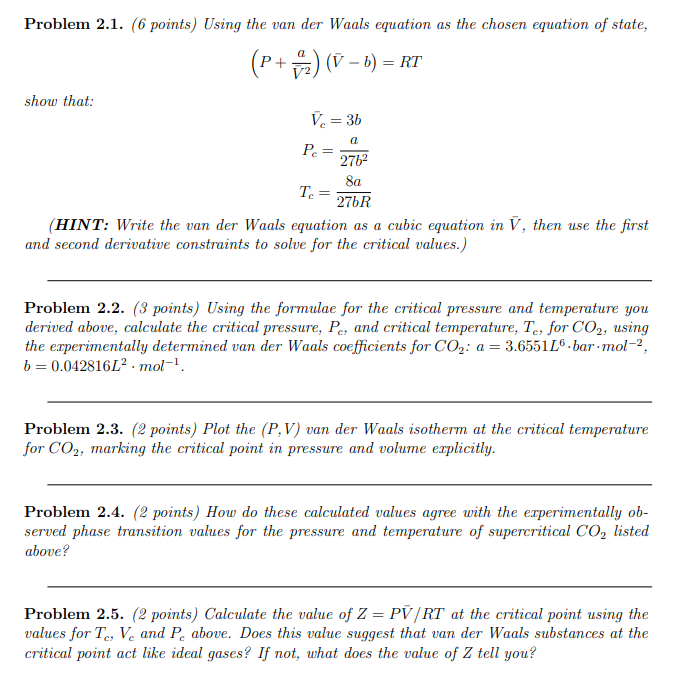
Solved Problem 2.1. (6 points) Using the van der Waals

38 1 THE PROPERTIES OF GASES Discussion PDF, PDF, Gases
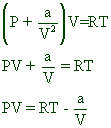
REAL GASES, DEVIATION FROM IDEAL GAS BEHAVIOUR
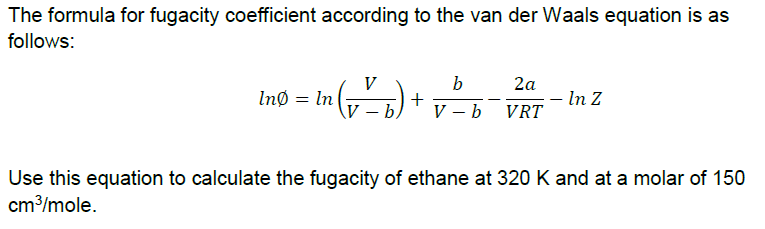
Solved The formula for fugacity coefficient according to the
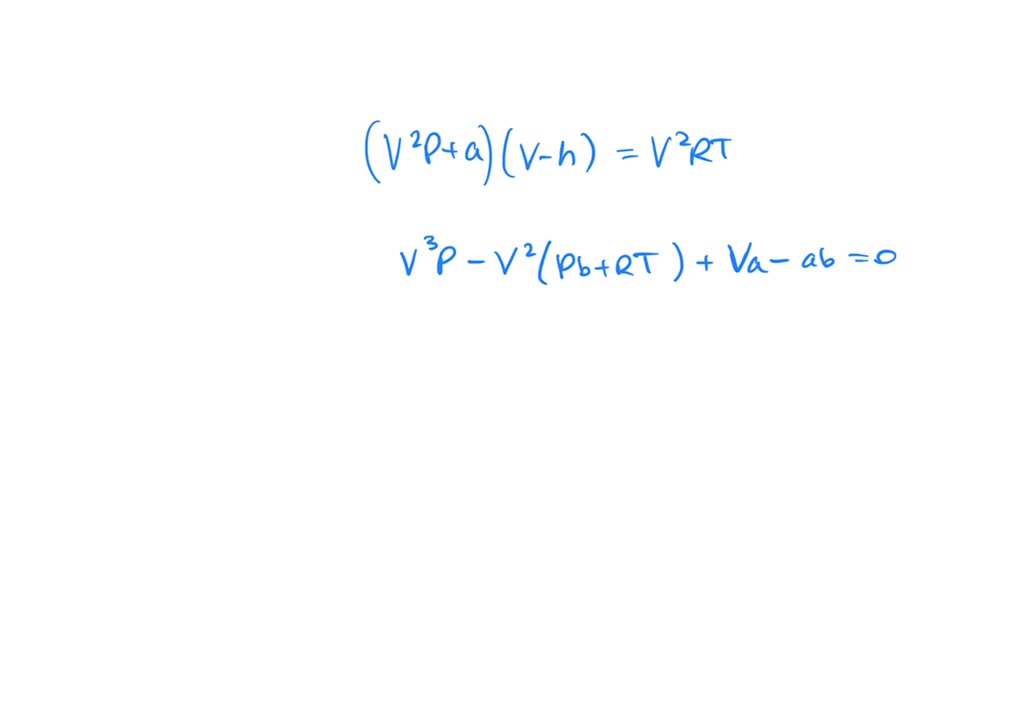
SOLVED: Van der Waals Equation of state is given, where P is the pressure (10 atm), T is the temperature (300 K), R is the Ideal Gas Constant (0.08206 L.atm/mol.K), a is
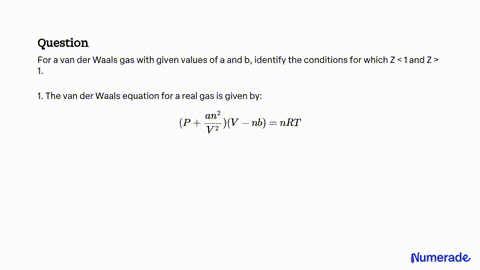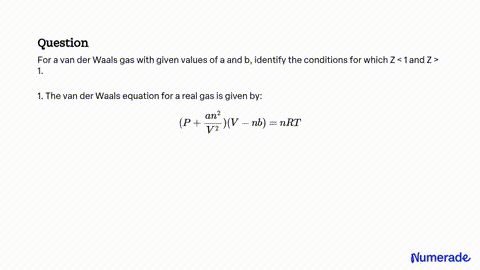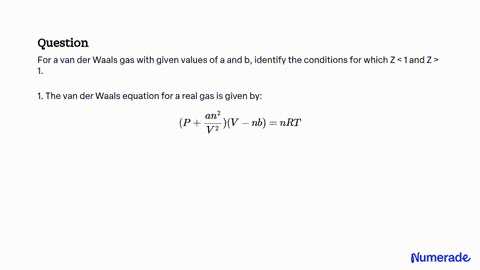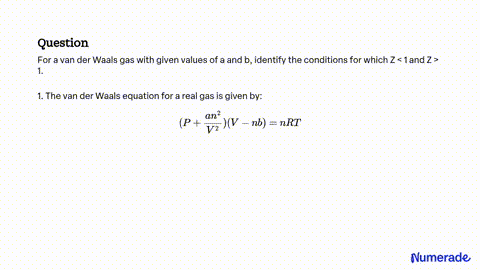
SOLVED: For a van der Waals gas with given values of a and b, identify the conditions for which Z<1 and Z>1


Peter Atkins Julio de Paula Ron Friedman Physical Chemistry Quanta (0358-0408), PDF, X Ray Crystallography

⏩SOLVED:If Z is a compressibility factor, van der Waals equation at…
Solved (15 pts) The van der Waals equation of state is

CH-Physical Chemistry(8th ed)[英语]Atkins

SOLVED: Use the van der Waals Eq. in terms of reduced quantities to derive the condition for Z > 1, and Z < 1, respectively. Use an expression of TR in terms of VR.
- At Critical Temperature,pressure and volume . The compressibility Factor (Z) Is
- Derive an expression for the compression factor of a gas tha
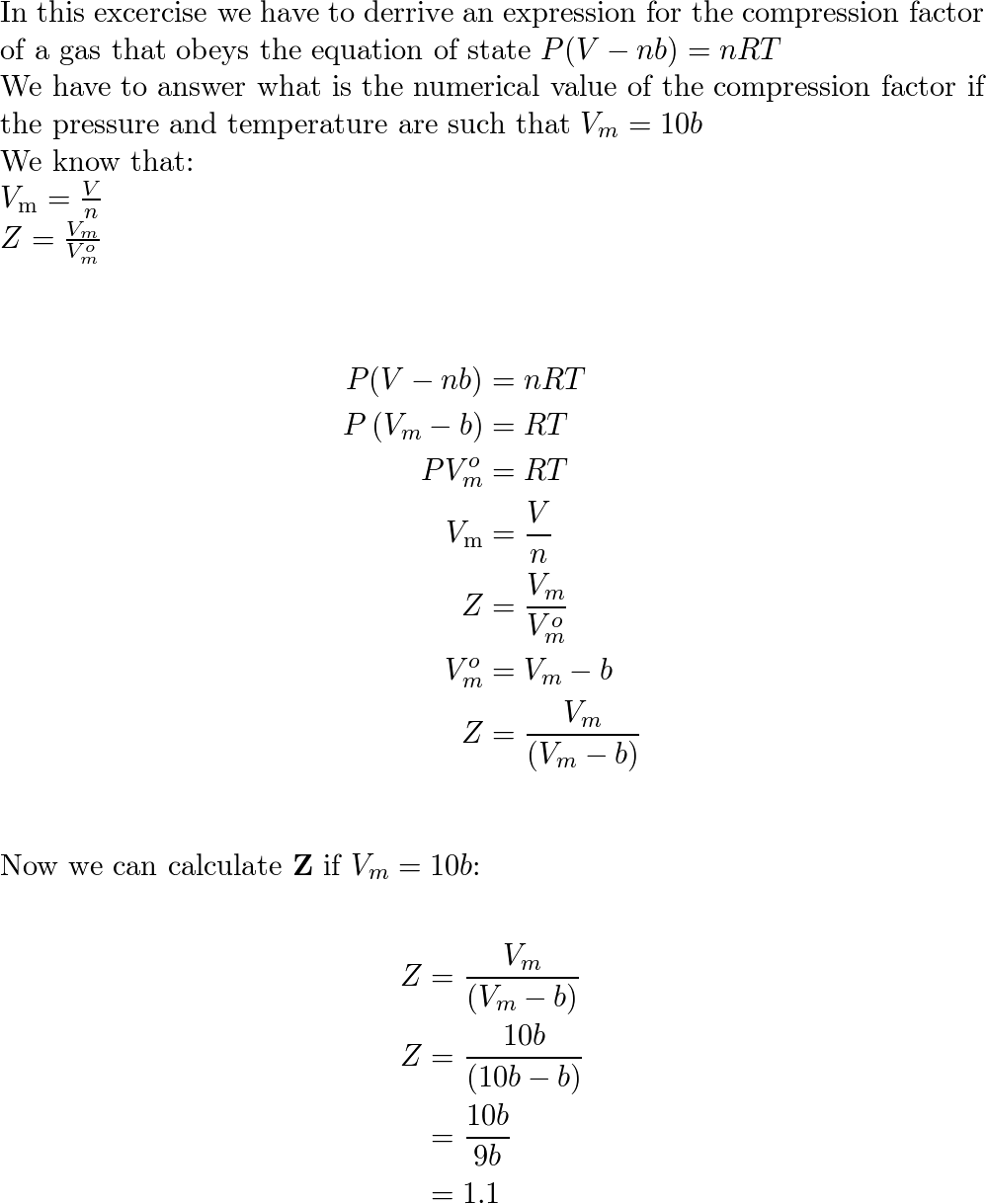
- At 273 K measurements on argon gave B = -21.7 cm$^3$ mol$^{

- The Compression Factor, Z, and Real Gases - What you NEED to Know!

- SOLVED: For a gas at a given temperature, the compression factor




.jpg)
