Tuesday, Oct 01 2024
Solved 1. The compression factor, Z of a gas is 0.625. Which
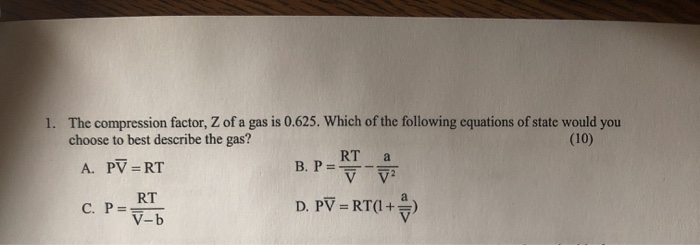
By A Mystery Man Writer

Simple Equation Real Gas Compressibility Factor Z

Compressibility factor, Z of a gas is given as `Z=(pV)/(nRT)` (i) What is the value of Z for an

What is compressibility factor? What is its value for ideal gas

PDF) Simple methods for the calculation of thermodynamic properties for metering
Solved] Sketch how the compression factor z of an ideal and real gases

Norriseal valve sizing reference guide by RMC Process Controls & Filtration, LLC. - Issuu

Physical Chemistry The Compression Factor (Z) [w/1 example]

Determine Compressibility of Gases

Equation of state and compressibility factor for the HCYF with ϭ 9.
Related searches
- How to Calculate Compression Ratio: 9 Steps (with Pictures)

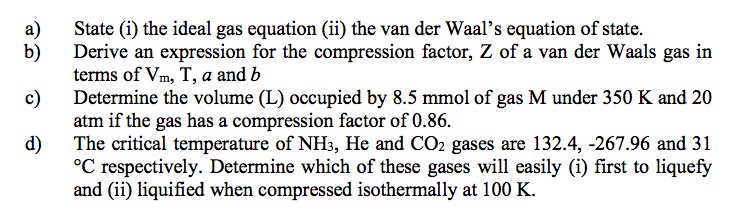
- Solved a) b) c) State (i) the ideal gas equation (ii) the
- Compression Factor and Fugacity

- Write an equation for the transformation of y=x vertical compression by a factor of 1/11

- Compression Factor Exam Problem using Molar Volumes - Fully Explained!

Related searches
- Flarixa Women High Waist Trainer Body Shaper Panty Women Flat Belly Shaping Panties Tummy Control Underwear Butt Lifter Panties

- Editorial Universal Standard

- CRZ YOGA Women's Naked Feeling Soft Yoga Leggings 21 Inches - Yoga

- Branded Bra Name

- Hanes, The Unisex 7.8 oz., Ecosmart® 50/50 Crewneck Sweatshirt - HEATHER RED - M

©2016-2024, changhanna.com, Inc. or its affiliates