10.4: The Ideal Gas Equation - Chemistry LibreTexts

By A Mystery Man Writer
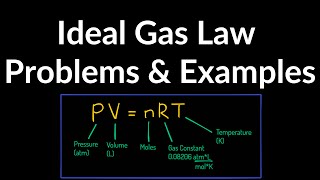
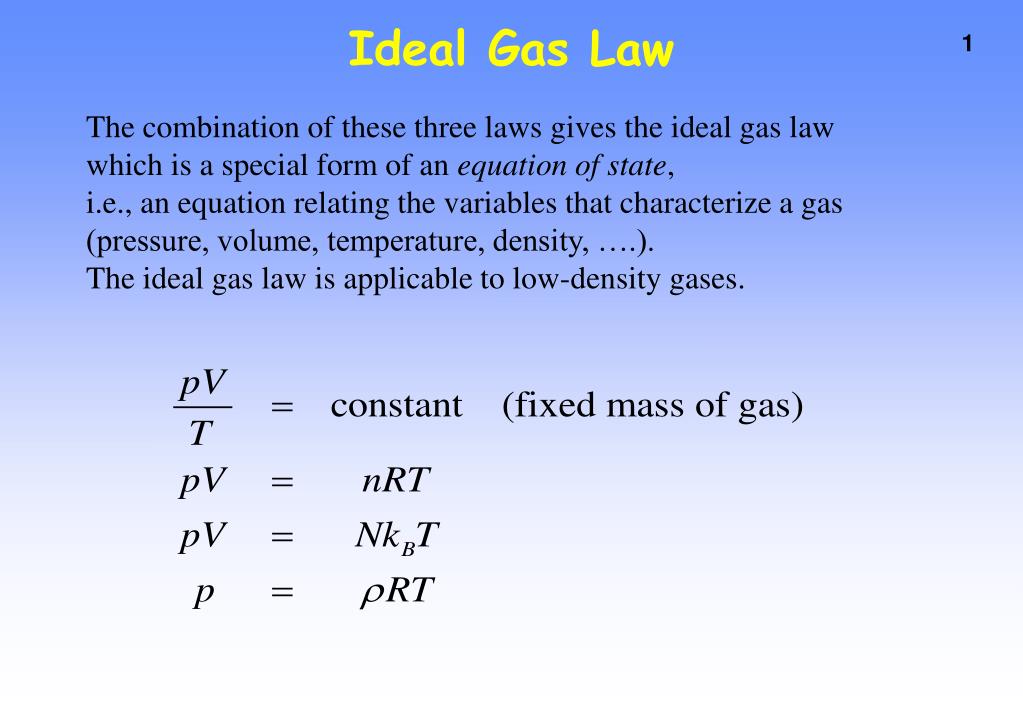
The empirical relationships among the volume, the temperature, the pressure, and the amount of a gas can be combined into the ideal gas law, PV = nRT. The proportionality constant, R, is called the …
The empirical relationships among the volume, the temperature, the pressure, and the amount of a gas can be combined into the ideal gas law, PV = nRT. The proportionality constant, R, is called the gas constant. The ideal gas law describes the behavior of an ideal gas, a hypothetical substance whose behavior can be explained quantitatively by the ideal gas law and the kinetic molecular theory of gases. Standard temperature and pressure (STP) is 0°C and 1 atm.

10.5: Further Applications of the Ideal-Gas Equations - Chemistry LibreTexts

The Ideal Gas Law - Chemistry LibreTexts, PDF, Gases

Foods, Free Full-Text

The Ideal Gas Law - Chemistry LibreTexts, PDF, Gases
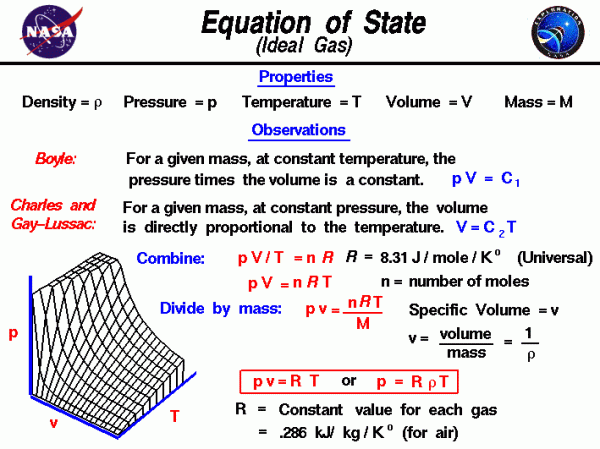
Equation Of State (Ideal Gas), Glenn Research Center
The Ideal Gas Law Boundless Chemistry
qph.cf2.quoracdn.net/main-qimg-63d40936e96331b4444

The Ideal Gas Law - Chemistry LibreTexts, PDF, Gases

Gas « KaiserScience

487928109-Physical-Chemistry-McQuarrie-and-Simon-Full.pdf

10.3: Relating Pressure, Volume, Amount, and Temperature- The Ideal Gas Law - Chemistry LibreTexts
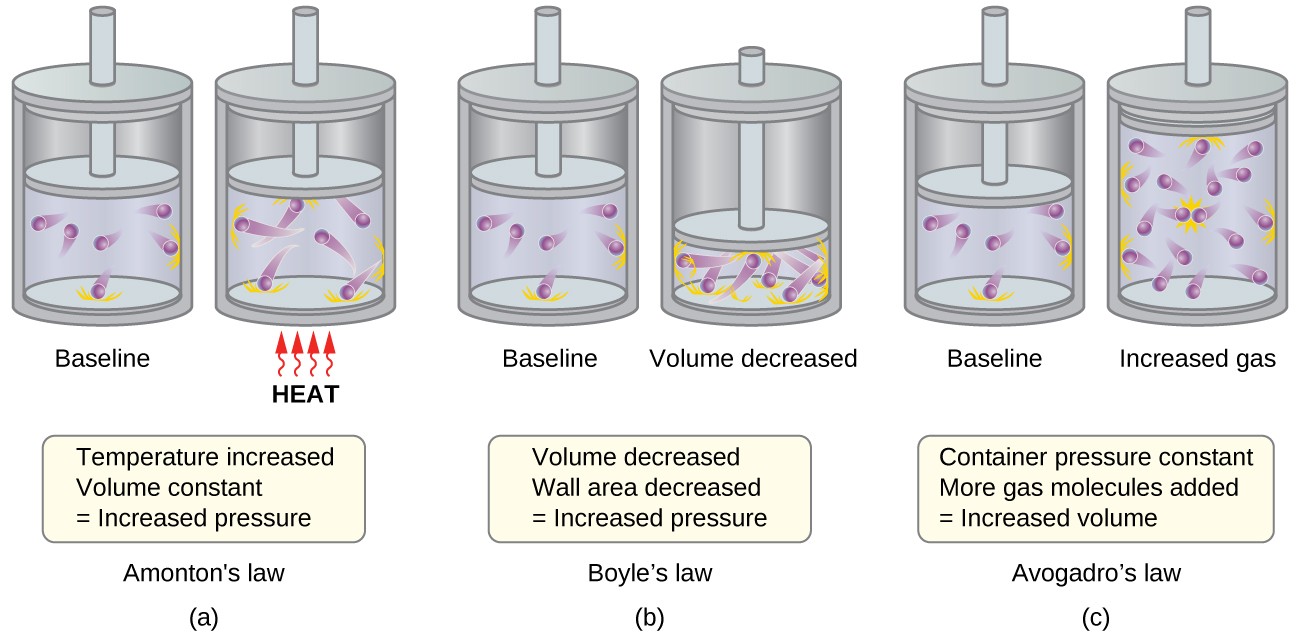
9.7 The Kinetic-Molecular Theory – Chemistry Fundamentals

Introduction to the Physics of Atoms, Molecules and Photons

8.1 Chemical Equations and Stochiometric Relationships – Chemistry Fundamentals
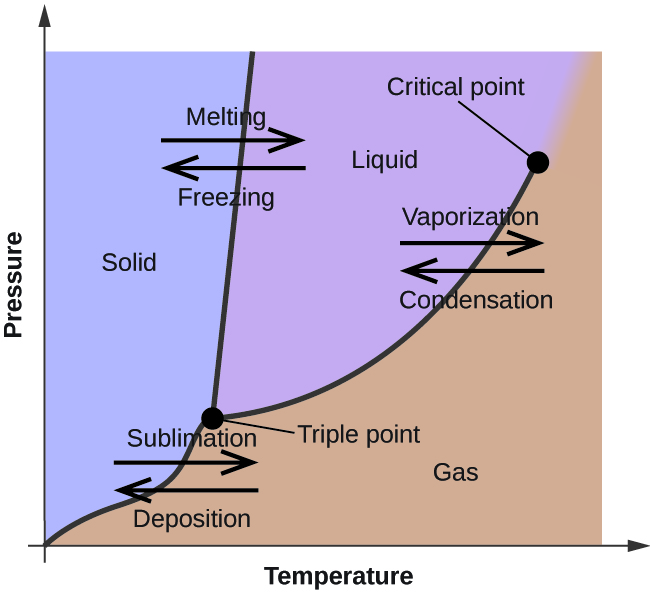
11.5 Phase Diagrams – Chemistry Fundamentals
- Wireless Womens Lace Bras Plus Size No Underwire Everyday Bra Full Coverage Memory Touch Brassieres Basic Bralettes

- Natori Flora Contour Underwire Bra- Alpine

- Meia arrastão - CORES - Modarce

- BB Branded, Windsor
- Exercise & Fitness: Sports & Outdoors: Clothing, Running, Yoga, Strength Training Equipment & More


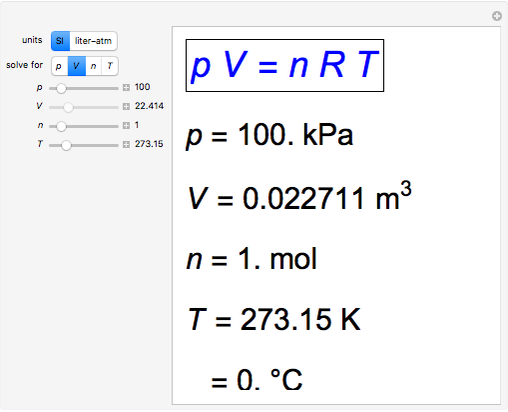
:max_bytes(150000):strip_icc()/200175879-001-56a12e6b5f9b58b7d0bcd67f.jpg)
