The compression factor (compressibility factor) for one mole of a Van der..

By A Mystery Man Writer
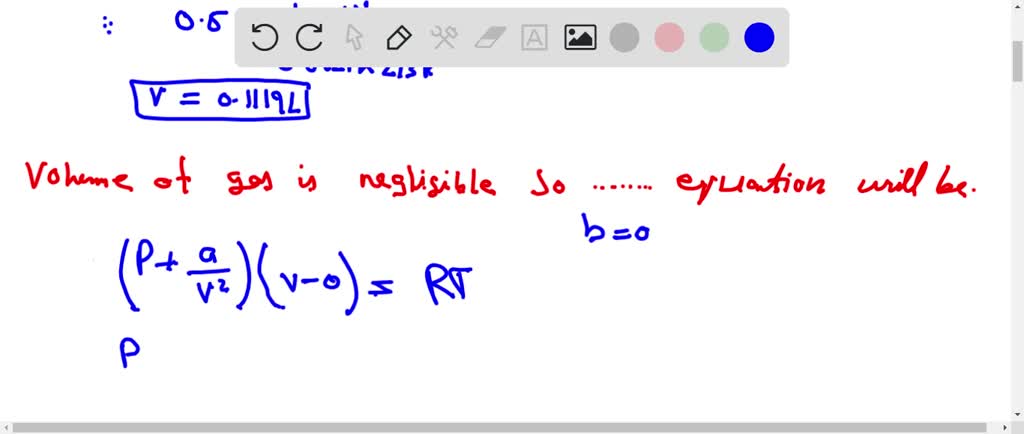
Solution For The compression factor (compressibility factor) for one mole of a Van der Waals' gas at 0∘C and 100 atmosphere pressure is found to be 0.5 . Assuming that the volume of a gas molecule is
The compression factor (compressibility factor) for one mole of a Van der Waals' gas at 0∘C and 100 atmosphere pressure is found to be 0.5 . Assuming that the volume of a gas molecule is negligible, calculate the Van der Waals' constant 'a',
[JEE 2001]
ns. 1.256 atm L2 mol−2
Z=1−VmRTaZ=RTPVm⇒Vm=PRTZZ=1−(RT)2a×ZP0.5=1−(273×0.0821)2×.5a×100a=1.256 atm L2 mol−2
Video solution 1: The compression factor (compressibility factor) for one mole of a Van der Waals' gas at 0∘C and 100 atmosphere pressure is found to be 0.5 . Assuming that the volume of a gas molecule is negligible, calculate the Van der Waals' constant 'a',
[JEE 2001]
ns. 1.256 atm L2 mol−2
Z=1−VmRTaZ=RTPVm⇒Vm=PRTZZ=1−(RT)2a×ZP0.5=1−(273×0.0821)2×.5a×100a=1.256 atm L2 mol−2

The compression factor (compressibility factor) one mole of a van

Poulduly 59. What is the compressibility fac is the

Compressibility factor - Wikipedia

At a high pressure, the compressibility factor (Z) of a real gas is us
⏩SOLVED:The compression factor (compressibility factor) for one

The compression factor (compressibility factor) for `1 mol` of a

18. The compressibility factor one mole of a vanderwaal's gas 0°C

al Gases f.a What is the compressibility factor (Z) 0.02 mole of a
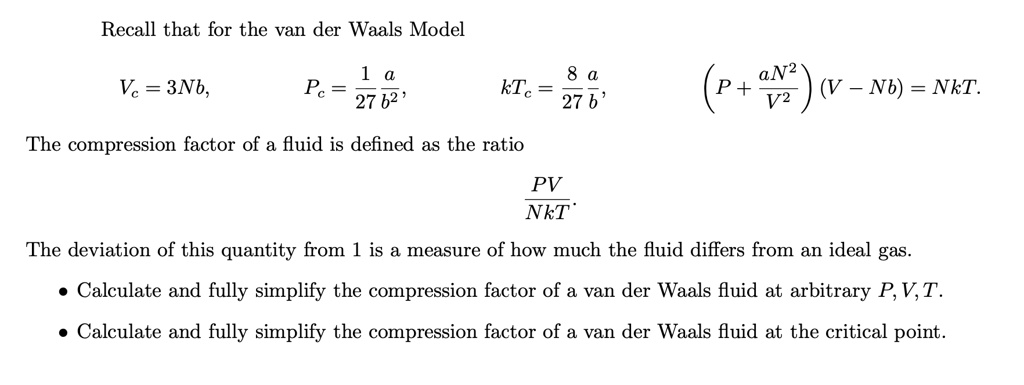
SOLVED: Recall that for the van der Waals Model: Vc = 3Nb, Pc
The compression factor (compressibility factor) for one mole of a

SOLVED: (d) What is compressibility factor Ind the Boyle

Bengali] The compressibility factor (Z) of one mole of a van der Waal

Welcome to Chem Zipper.com: The compressibility factor for 1

Non-Ideal Gas Behavior Chemistry: Atoms First

At high pressure, the compressibility factor for one mole of van der w
- Solved Show that the compressibility factor of van der Waals

- 1. The compressibility factor, z, is the ratio of

- PDF] COMPARISON OF FIVE NATURAL GAS EQUATIONS OF STATE USED FOR FLOW AND ENERGY MEASURMENT

- Procedure calculates base gas compressibility factors
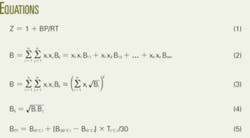
- Thermodynamics - 3-7 Ideal Gas Equation with compressibility





