Solved 14. The latent heat of vaporization of isopropyl

By A Mystery Man Writer
Answer to Solved 14. The latent heat of vaporization of isopropyl

SOLVED: Isopropyl alcohol has a heat of vaporization of 3.99 * 10^3 mol^-1 and a boiling point of 82.30 °C at 1.000 atm. Using the Clausius-Clapeyron equation, calculate the vapor pressure of
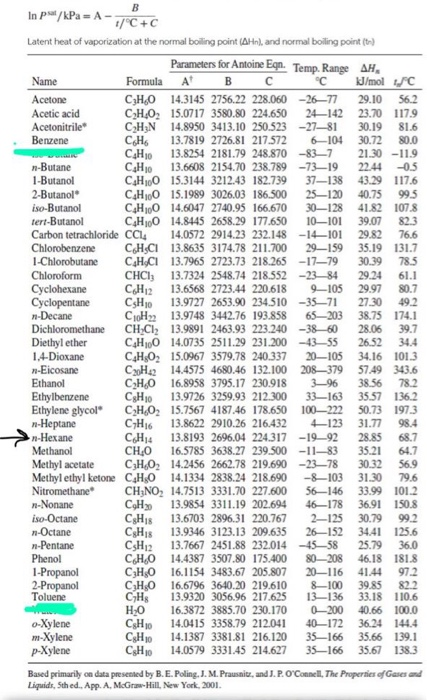
Solved Binary system 1-chlorobutane/chlorobenzene conforms
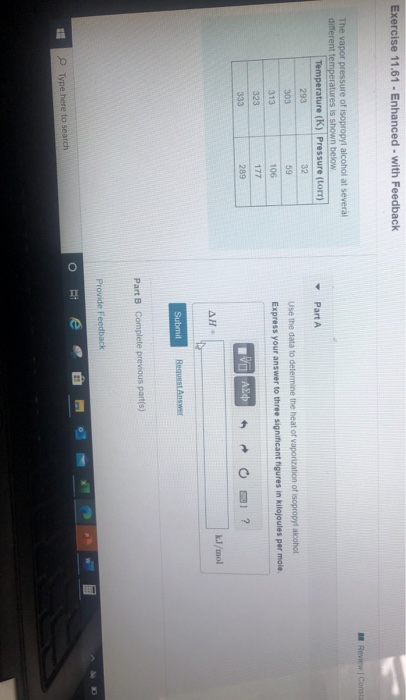
Solved Exercise 11.61 - Enhanced - with Feedback Review
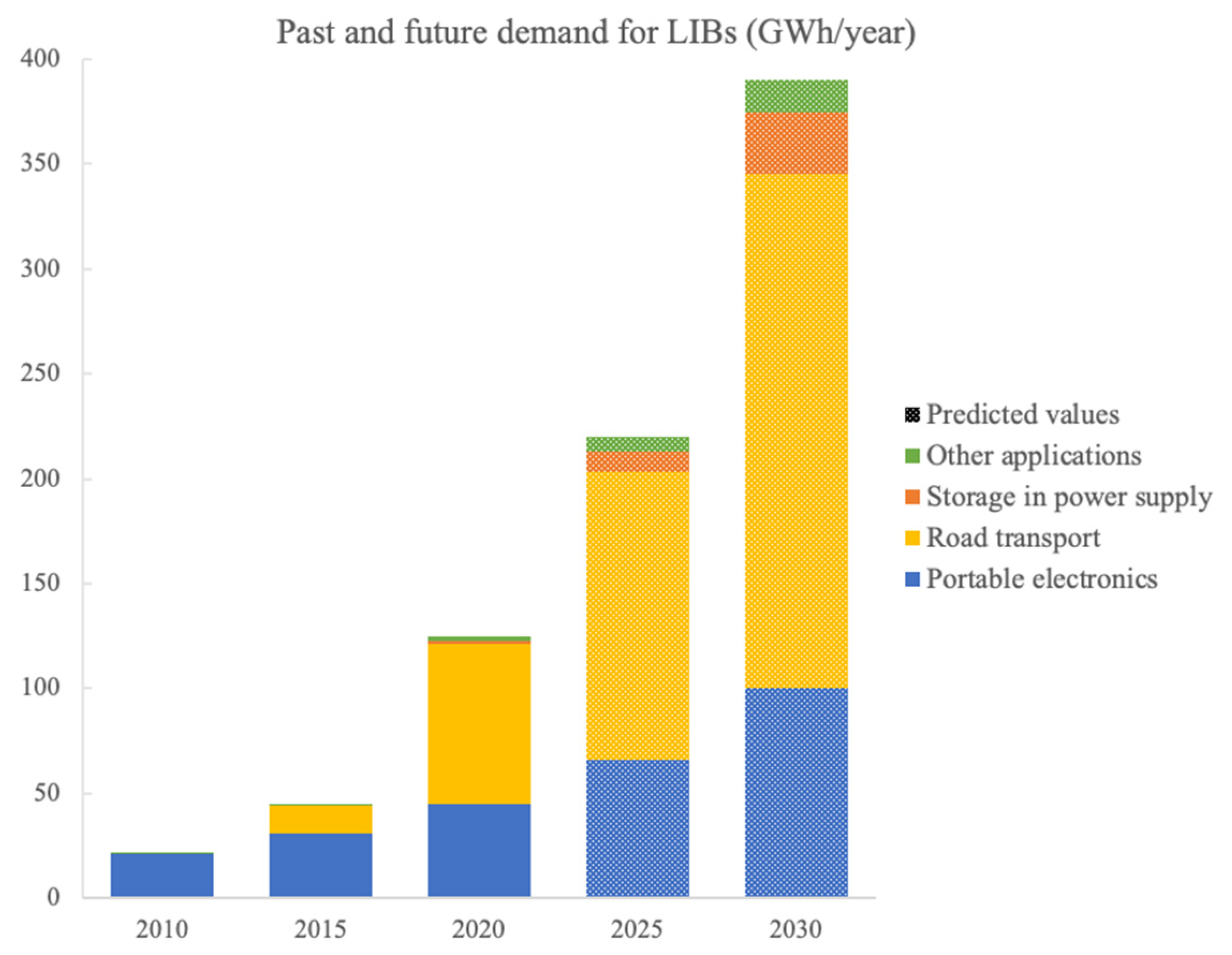
Energies, Free Full-Text
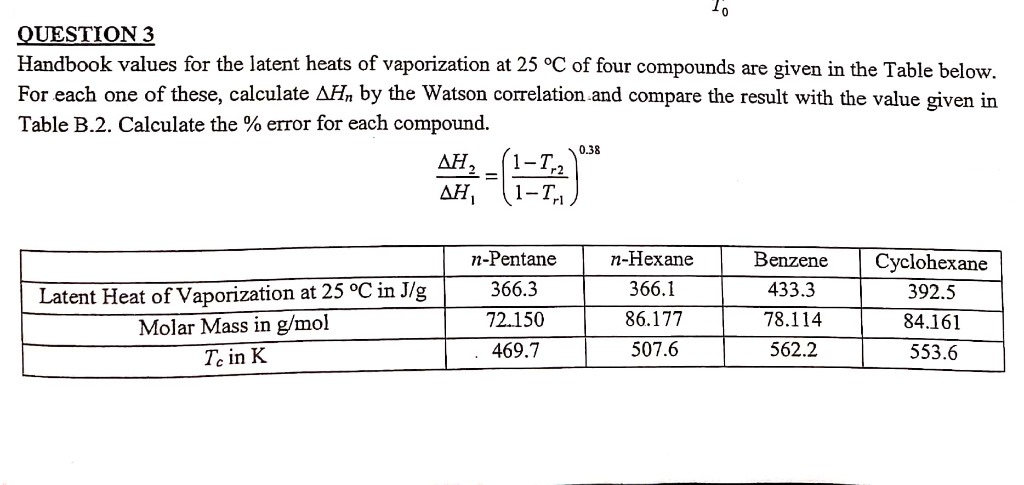
Solved UESTION 3 Handbook values for the latent heats of

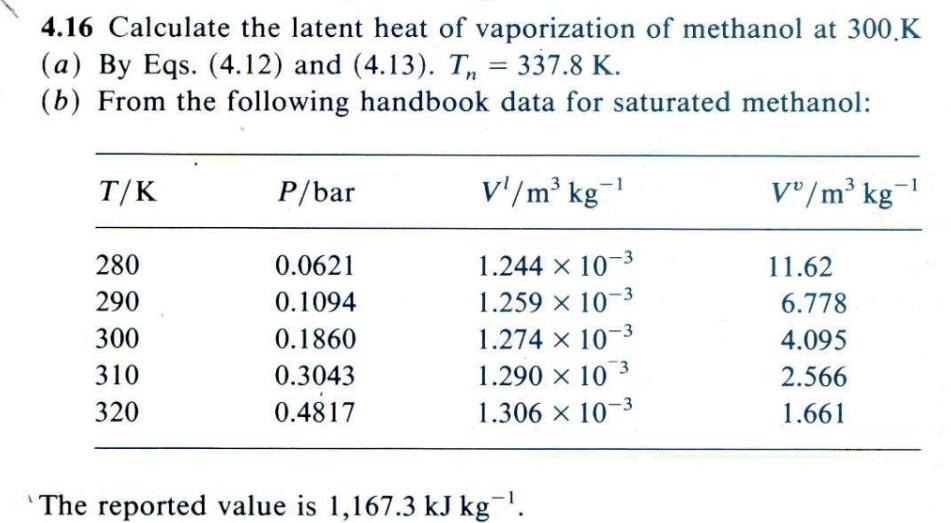
Solved AH RTn 1.092(In Pc - 1.013) 0.930 - Tr (4.13) 4.16
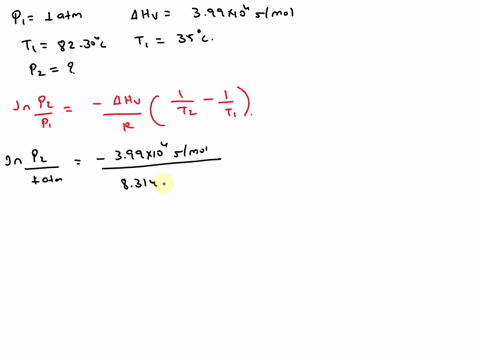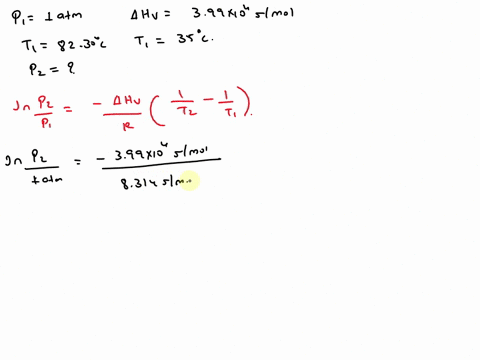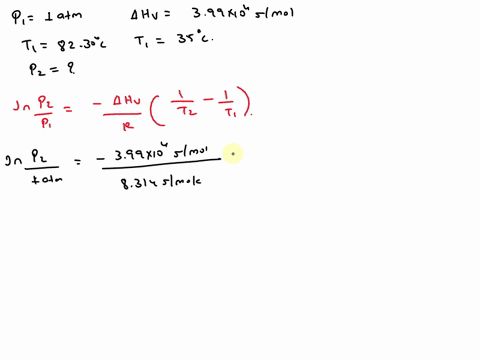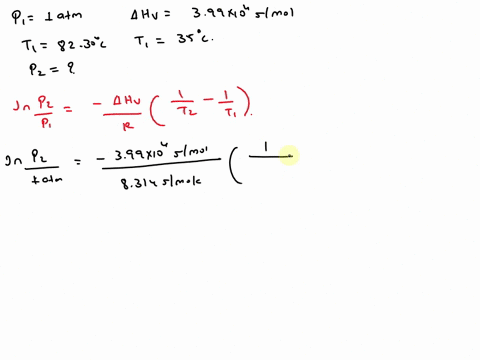
SOLVED: Isopropyl alcohol has a heat of vaporization of 3.99 * 10^3 mol^-1 and a boiling point of 82.30 °C at 1.000 atm. Using the Clausius-Clapeyron equation, calculate the vapor pressure of

Polymers, Free Full-Text
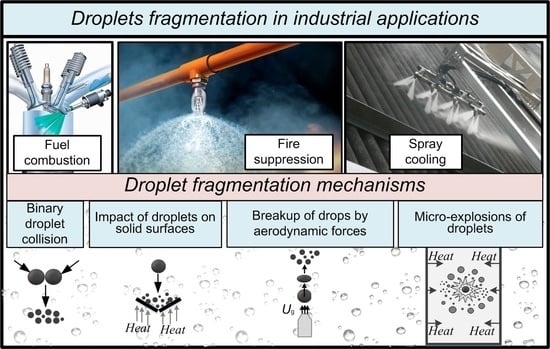
Energies, Free Full-Text
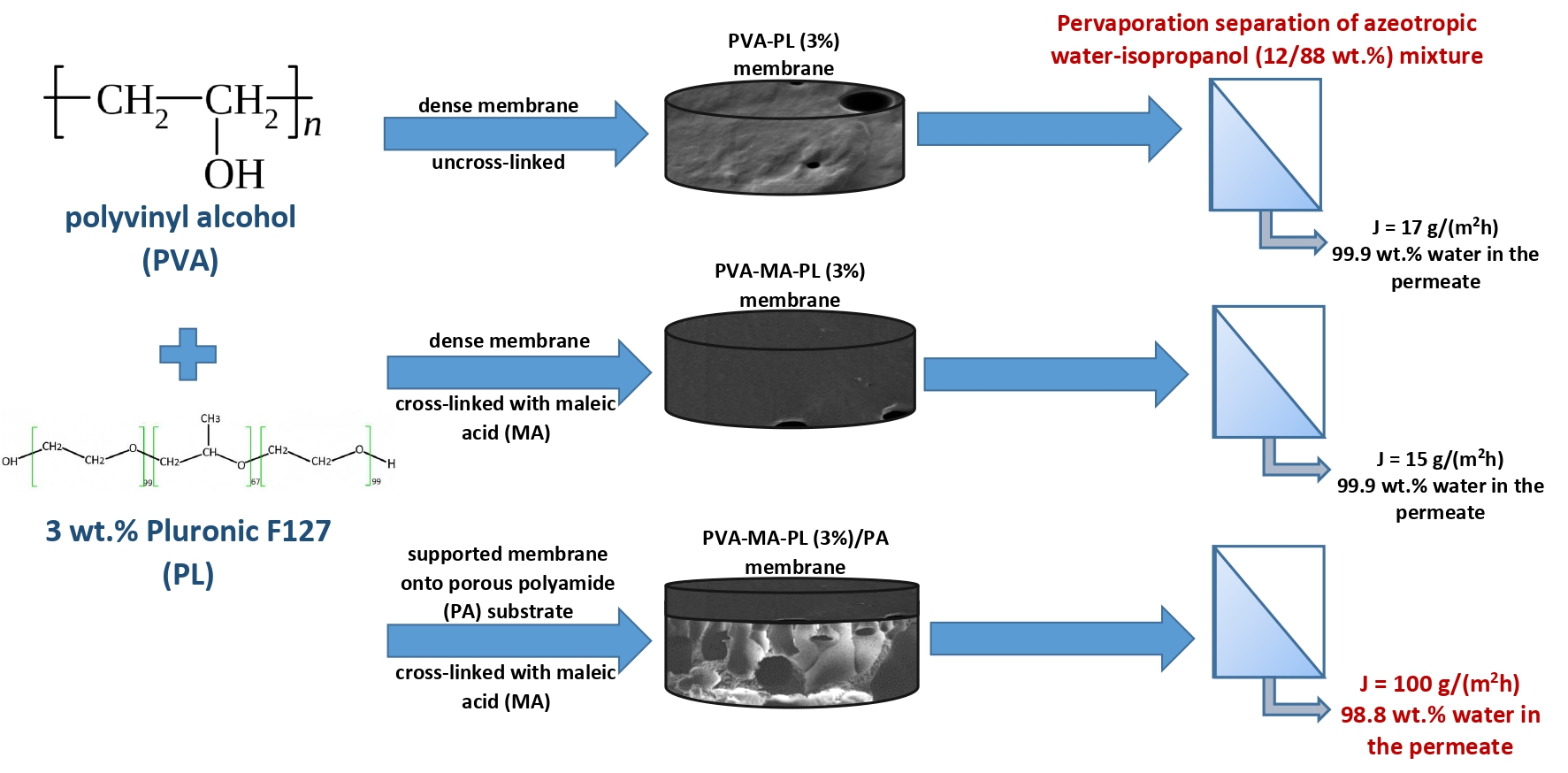
Sustainability, Free Full-Text
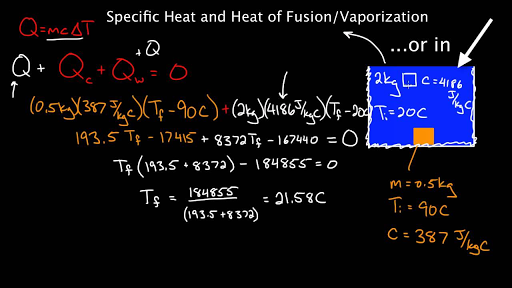
Specific heat and latent heat of fusion and vaporization (video)
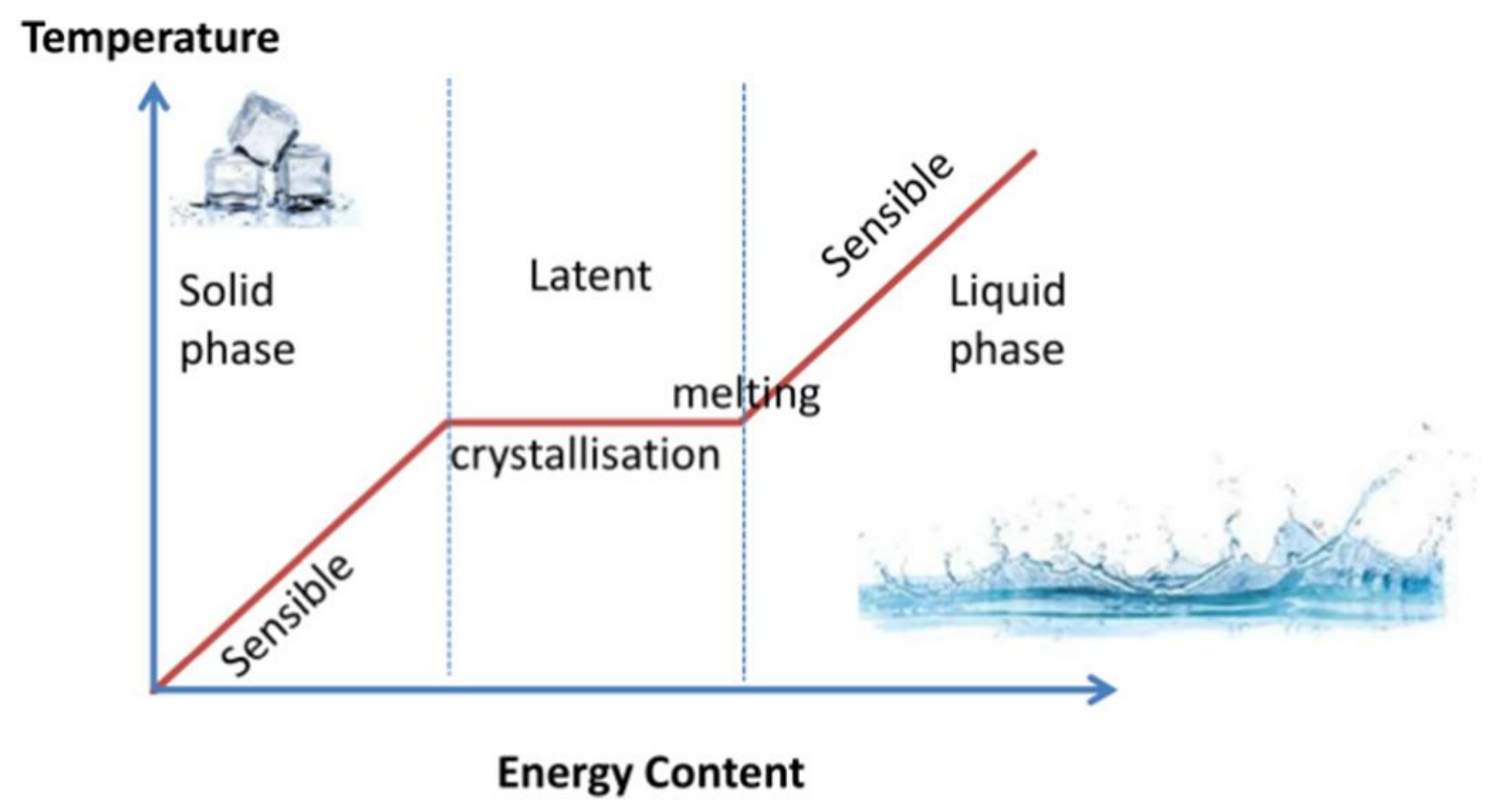
Energies, Free Full-Text
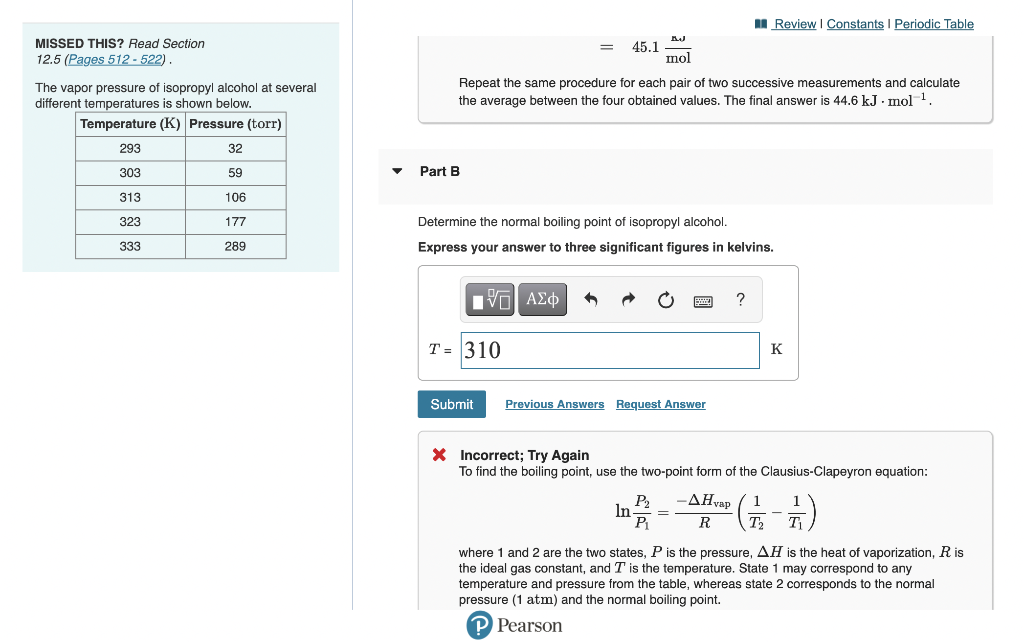
Solved I determined the heat of vaporization to be 44.6
- a) Type T thermocouple voltage to temperature conversion plot. (b

- Switching of linear polarization conversion as VO2 transitions

- How to Convert Temperature From Fahrenheit to Celsius

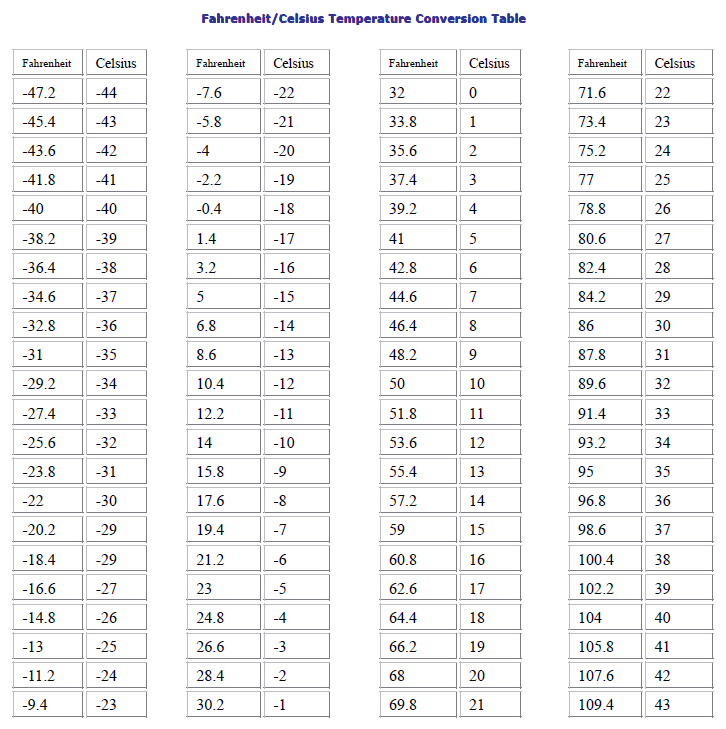
- Fahrenheit/Celsius Temperature Conversion Table-Technical Articles-Longer Precision Pump Co., Ltd.
- Trinx Kitchen Conversion Chart Framed On Canvas

- Sun Shirt Cropped Summer Shirt Cute Crop Top Sunshine Shirt Beach Shirt Cute Shirt for Women Summer Crop Tops Ocean Waves

- Shapewear Bodysuit for Women Tummy Control Full Body Shaper Open

- ELFINDEA Capri Pants for Women Fashion Floral Jumpsuit Sexy Strapless Jumpsuit Pink S

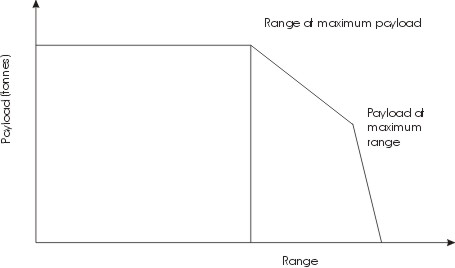
- Carga útil – Wikipédia, a enciclopédia livre
- Faja Invisible Short 2033 Smart Secret - CHIANTI JEANS
