Compressibility factor (Z) for a van der Waals real gas at

By A Mystery Man Writer
Share your videos with friends, family and the world

PDF) A Modified Form of the van der Waals Equation of State

Class Notes on Compressibility of a Real Gas, CH 417, Study notes Physical Chemistry

COMPREHENSION_TYPE from IIT-JEE PREVIOUS YEAR (CHEMISTRY) STATES OF MATTER for Class 12
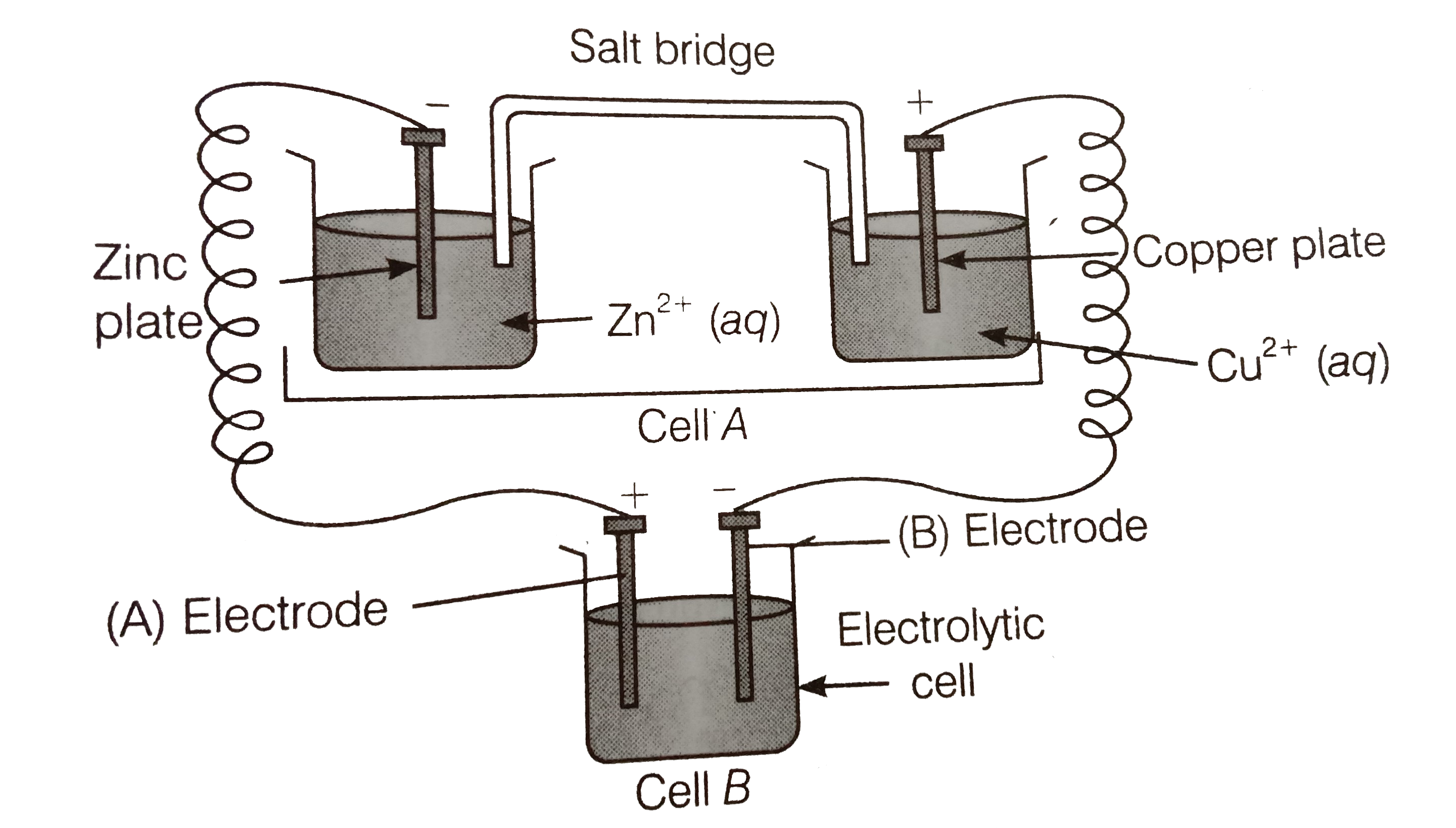
For the cell: A, A^(m+), B^(n+)

Non-Ideal Gas Behavior Chemistry: Atoms First

Comparison of various state equations for carbon dioxide at 9 MPa
For a certain van der Waal's gas, critical temperature is-243^(@)C. Ma

Deviation of Real Gases from Ideal Gas Behaviour - GeeksforGeeks
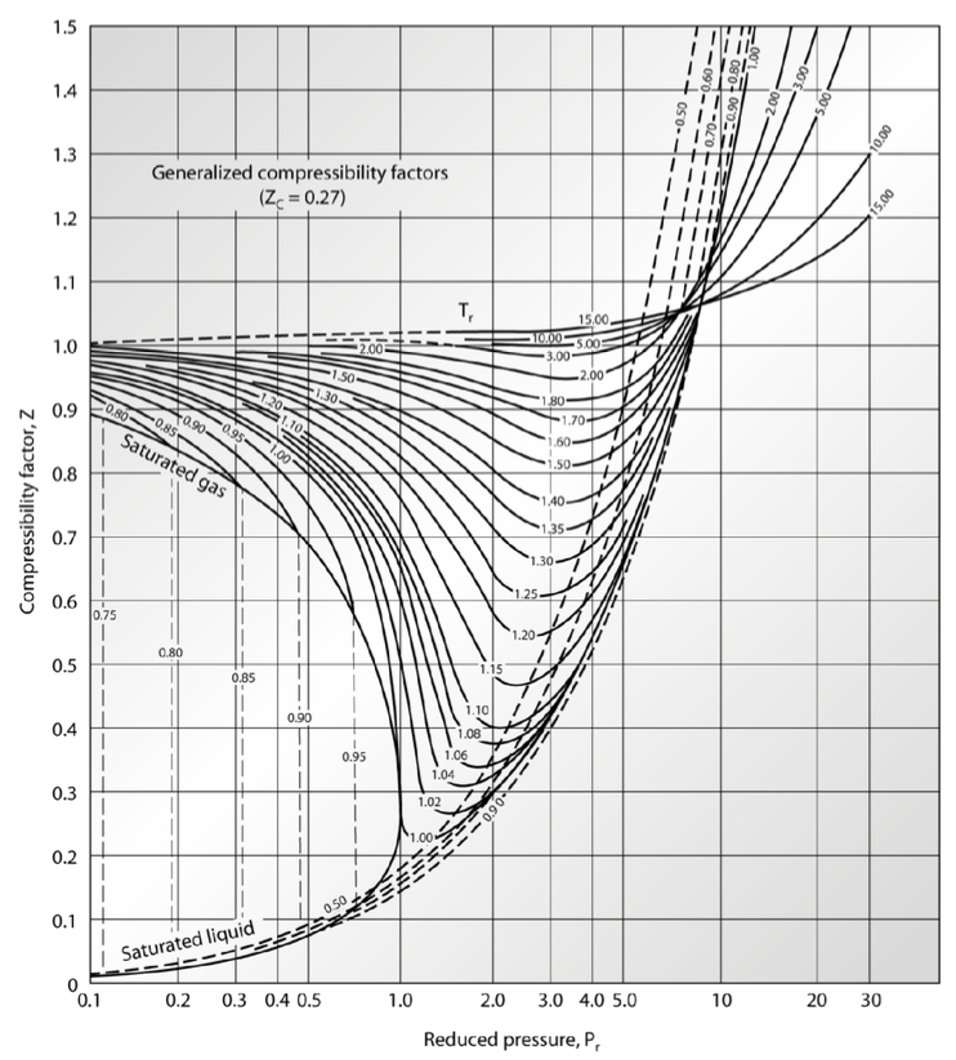
Qin Lab - thermal data

Compressibility factor, Z of a gas is given as Z=(pV)/(nRT) (i) What
Complete Solutions to Mock Test 1 of chapter MOCK TEST of Class 11 book with complete answers and questions
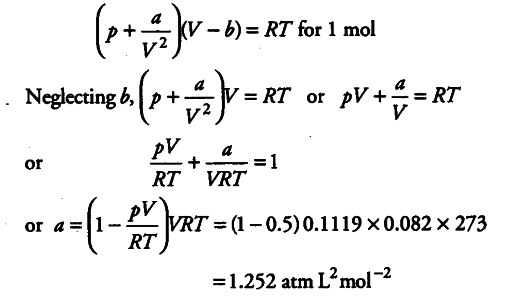
The compression factor (compressibility factor) for one mole of a van - CBSE Class 11 Chemistry - Learn CBSE Forum

Non-ideal behavior of gases (article)
- Compressibility Factor of Gas, Overview, Equation & Chart - Lesson

- Compressibility Factor Calculator
- The value of compression factor at the critical state of a vander waals gas is
- PPT - The Ideal Gas PowerPoint Presentation, free download - ID:6789672

- Solved] Why is the compressibility factor less than 1 at most




