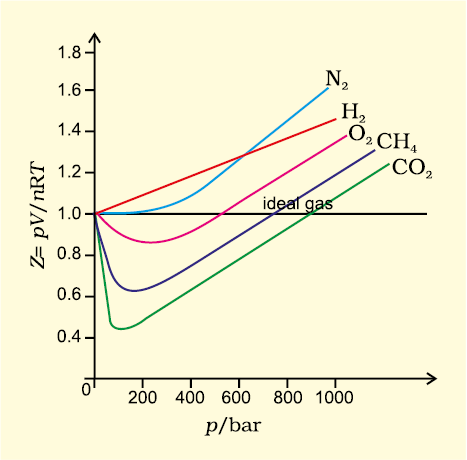
For a given gas, a graph is shown between compressibility factor

By A Mystery Man Writer
By the graph, at T2, the gas does not deviate from ideal gas behaviour at low pressure (Z = 1).Hence, T2 = TBT2 = TB (Boyle's temperature)T1 > TB (Here, Z > 1)This implies that T3 B and TB > TC(Here, Z So, T1 and T2 must be above TC.

gas laws - Graph of compressibility factor vs pressure when real gas is assigned Z=1 - Chemistry Stack Exchange

The given graph represents the variation of Z(compressibility factor =displaystyle frac{mathrm{P}mathrm{V}}{mathrm{n}mathrm{R}mathrm{T}}) versus mathrm{P}, three real gases mathrm{A}, mathrm{B} and C. Identify the only incorrect statement.For the gas C

The following graph is plotted between compressibility factor Z versus pressure of a gas at different temperatures.Which of the following statements is /are correct?
Sections

Van der Waals equation - Wikipedia

The given graph represents the variation of compressibility factor Z vs P for three gases A, B and C.Identify the incorrect statements.

Compressibility Factor - an overview
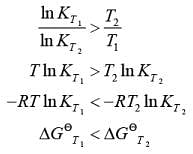
For a reaction,the plots of [A] and [P] with time at temperatures T1and T2are given below.If T2andgt;T1, the correct statement(s) is (are)(AssumeHandtheta;and Sandtheta;are independent of temperature and ratio of lnK at T1to

Deviation of Real Gases from Ideal Gas Behaviour - GeeksforGeeks

The given graph represents the variation of Z (compressibility factor) vs. P three real gases A, B and C. Identify the correct statementFor the gas A, a=0 and its dependence on P

Compressibility factor Z is plotted against pressure P for four different gases A , B , C & D. The correct order of critical temperature of the gases shown in the below

The following graph is plotted between compressibility factor Z versus pressure of a gas at different temperatures.Which of the following statements is /are correct?

The graph of compressibility factor (Z) vs. P for one mole of a real gas is shown in following d
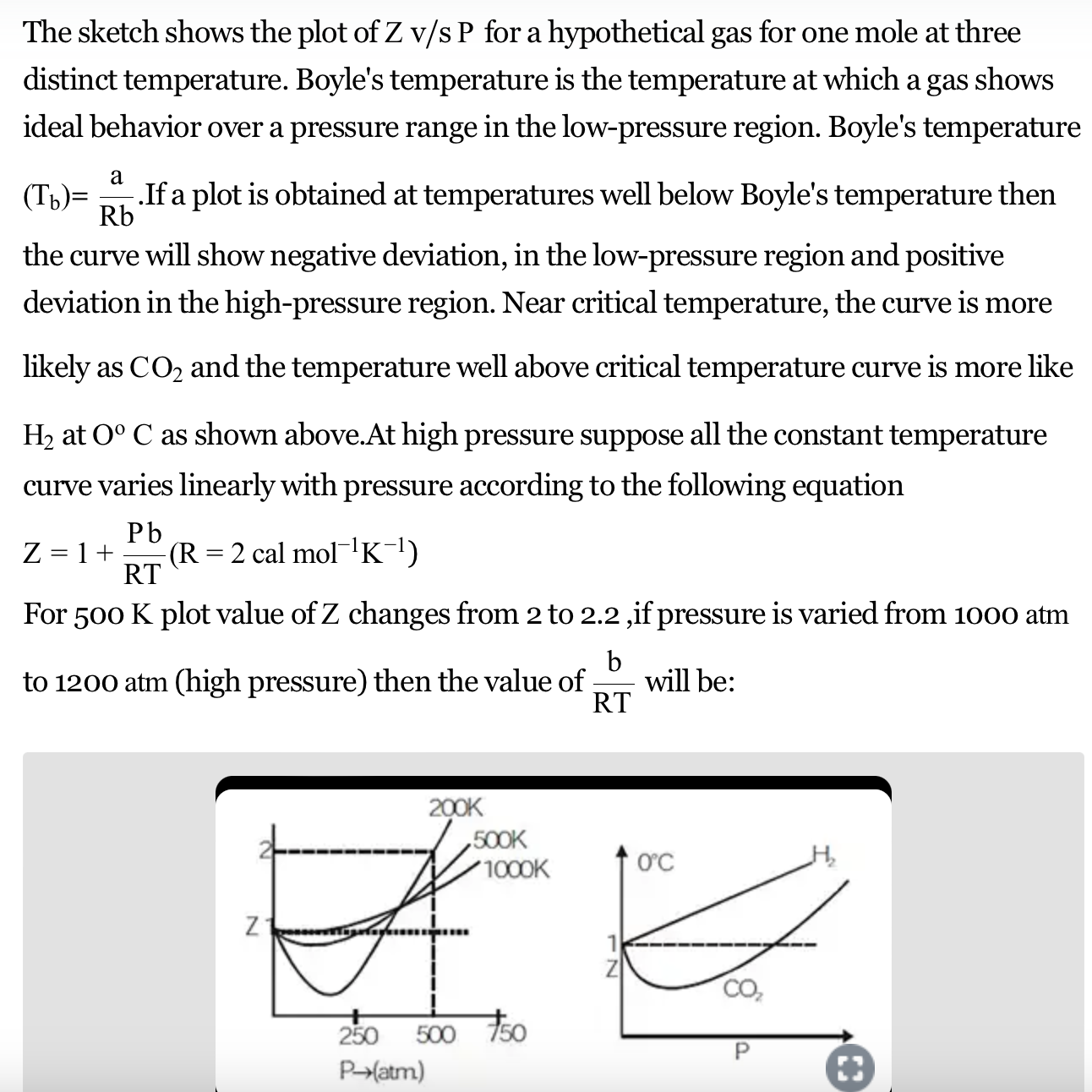
Solved The graph of compressibility factor (Z)v/sP for 1 mol
- Compressibility factor (gases) - Knowino
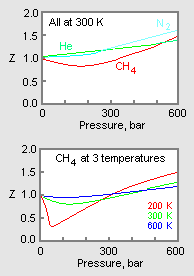
- Compressibility factor Z = PV / nRT is plotted against pressure as
- Compressibility Factor (Z) and pressure bar Stock Vector Image

- Determine Compressibility Factor with Present of CO2 and H2S
- For a given gas, a graph is shown between compressibility factor (Z) and Pressure (P).Select the incorrect statement(s) about the various temperature relations.a)Temperature T1 must be above critical temperature (TC).b)Temperature T2 may




/product/51/964049/1.jpg?4394)

