The vapour pressure of a solution having 2.0 g of solute X (gram atomic mass=32 g/mol) in 100 g of CS2 (vapour pressure =854torr) is 848.9 torr.The molecular formula of solute 1)
By A Mystery Man Writer
The vapour pressure of a solution having 2.0 g of solute X (gram atomic mass=32 g/mol) in 100 g of CS2 (vapour pressure =854torr) is 848.9 torr.The molecular formula of solute 1) X 2)X2 3)X4 4)X8
The vapour pressure of a solution having 2-0 g of solute X -gram atomic mass-32 g-mol- in 100 g of CS2 -vapour pressure -854torr- is 848-9 torr-The molecular formula of solute 1- X 2-X2 3-X4 4-X8
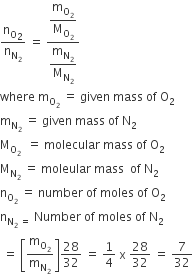
The vapour pressure of acetone at 20oC is 185 torr. When 1.2 g o

Solved 1. Assuming Raoult's Law applies, calculate the vapor
How to calculate the vapour pressure lowering caused by the addition of 200g of sucrose (mol mass=342) to 500g of water if the vapor pressure of pure water at 25 degrees Celsius
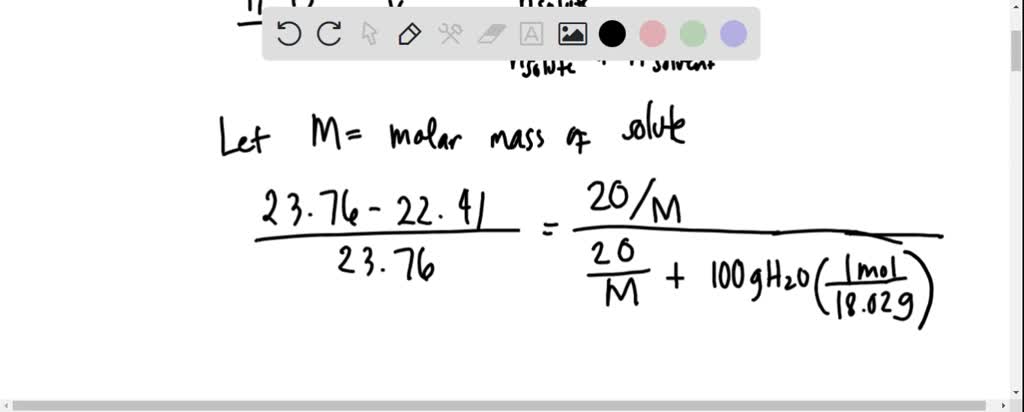
SOLVED: 20 g of solute was added to 100 g of water at 25^oC . The vapour pressure of water and that of solution were 23.76 mmHg and 22.41 mmHg respectively at

The vapour pressure of CS(2) at 50^(@)C is 854 torr and a solution o
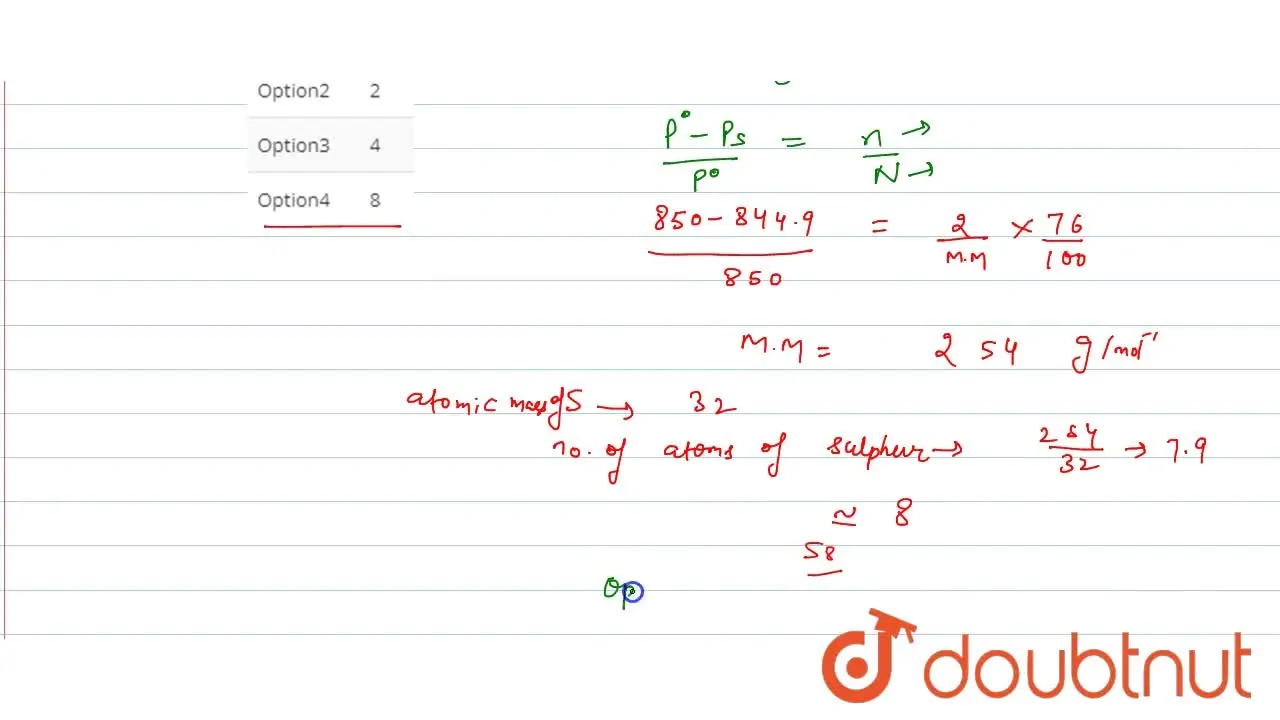
At 48^(@)C, the vapour pressure of pure CS(2) is 850torr . A solution

Solutions (Colligative Properties, Abnormality in Molar Mass) The vapour pressure of a solution having 2.0 g of solute X (gram atomic mass = 32 g mol -1) in 100 g of CS, (vapour

The vapour pressure of a solution having 2.0 g of a solute X( molar mass ..

The vapour pressure of `CS_(2)` at `50^(@)C` is `854 torr` and a solution of `2.0 g` sulphur in

Chapter-10 Solutions.pdf - Chemistry - Notes - Teachmint
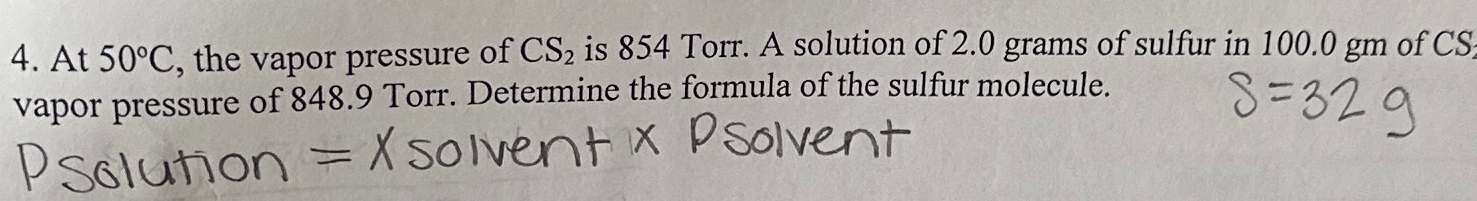
Solved At 50°C, the vapor pressure of CS2 is 854 Torr. A
The vapour pressure of a solution having 2.0 g of solute X (gram atomic mass =32 g/mol) in 100 g of CS2 (vapour pressure =854torr) is 848.9 torr.The molecular formula of solute 1)

Solutions (1-47) - Final, PDF, Solubility

the vapour pressure of 2% aqueous solution of a non volatile substance X at 373 k is 755 torr . Calculate

Coliyat 22. The vapour pressure of a solution having 2.0 g of solute X (gram atomic mass = 32 g mol-') in 100 of CS, (vapour pressure = 854 torr) is 848.9
- Comprar Chocolate Trento Duo 32G Peccin

- Moto G Pure 32 GB deep indigo 3 GB RAM

- Cubot J10, smartphone, Android 11, tela de 4 polegadas, telefones celulares MINI, 32 GB de RAM, Dual SIM 3G, identificação facial, bateria de 2350 mAh, câmera traseira de 5 MP, celular barato com frete grátis

- HALLS Rebuçados Energy Cola 32 g

- Rebuçados de Ice Tea sem Açúcar embalagem 32 g · Halls Energy