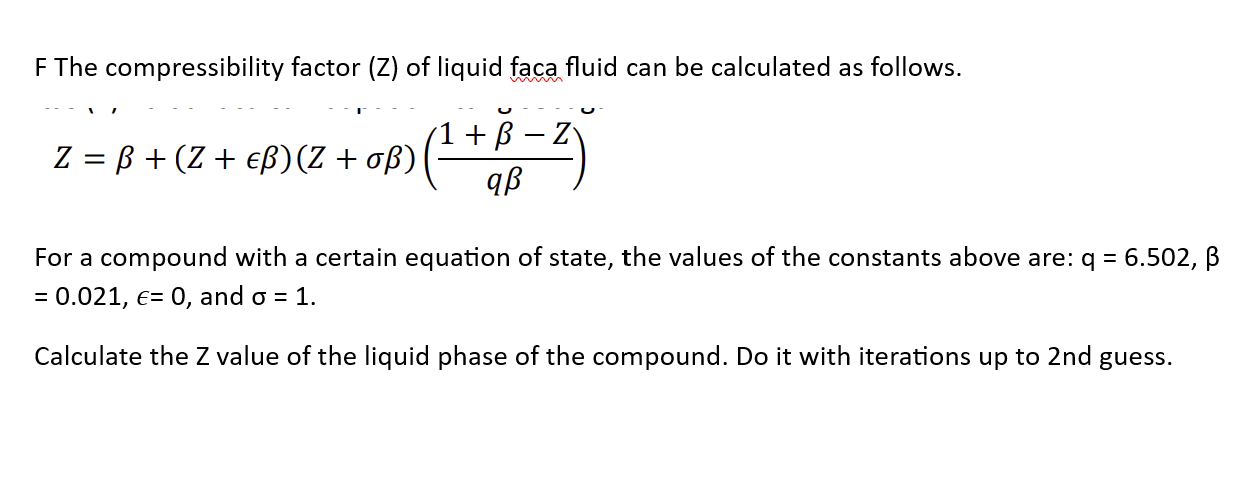
For $CO$, isotherm is of the type as shown. Near the point compressibility factor $Z$ is?\n \n \n \n \n 1.$\\left( {1 + \\dfrac{b}{V}} \\right)$ 2.$\\left( {1 - \\dfrac{b}{V}} \\right)$3.$\\left( {1 + \\

By A Mystery Man Writer
For $CO$, isotherm is of the type as shown. Near the point compressibility factor $Z$ is?\n \n \n \n \n 1.$\\left( {1 + \\dfrac{b}{V}} \\right)$ 2.$\\left( {1 - \\dfrac{b}{V}} \\right)$3.$\\left( {1 + \\dfrac{a}{{RTV}}} \\right)$4.$\\lef

Real Gas Behavior The Compression Factor (Z) [Example #2]

Solved NAME: 1.(a) Plot compression factor Z verses pressure

Compressibility factor - Wikipedia

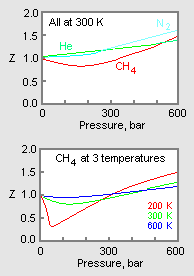
2. fluids 2
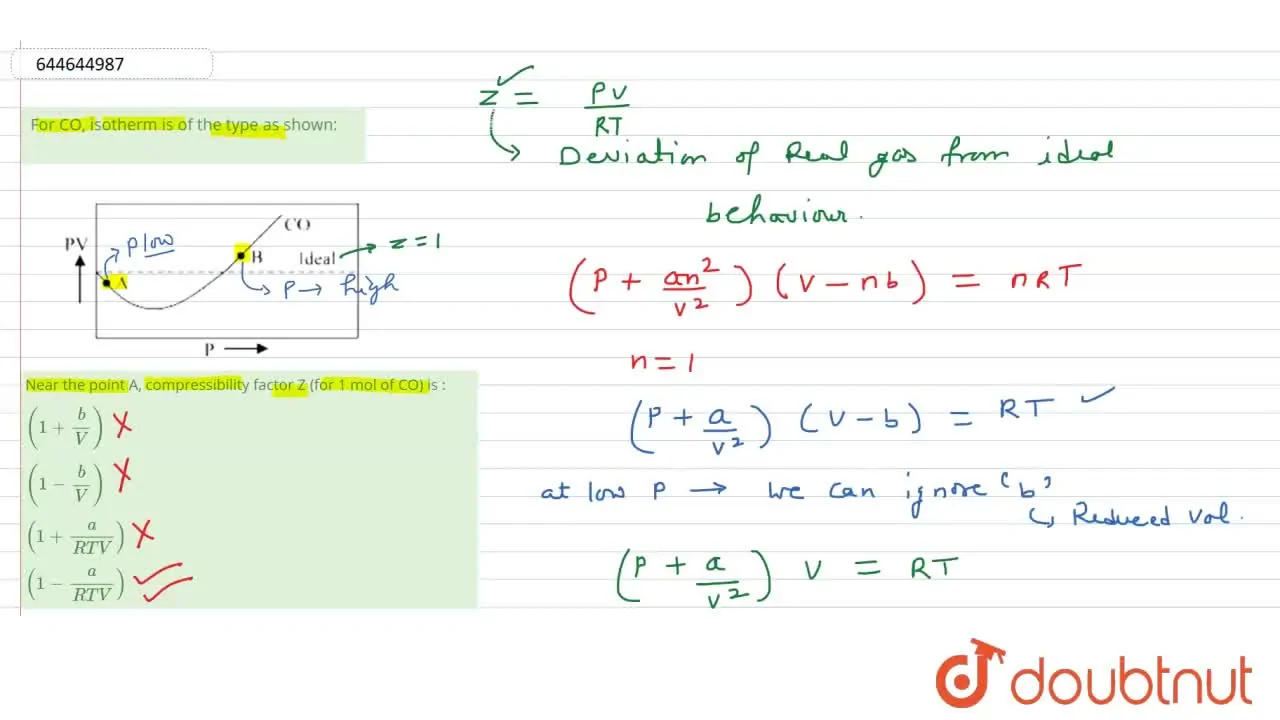
For CO, isotherm is of the type as shown: Near the point A, compr

Gas compressibility factor Z: Ideal gas vs Real gas

Gas compressibility factor Z: Ideal gas vs Real gas

The value of compressibility factor at the critical state the gas matches with the `Z_(c )` is

Real Gas Behavior The Compression Factor (Z) [Example #2]

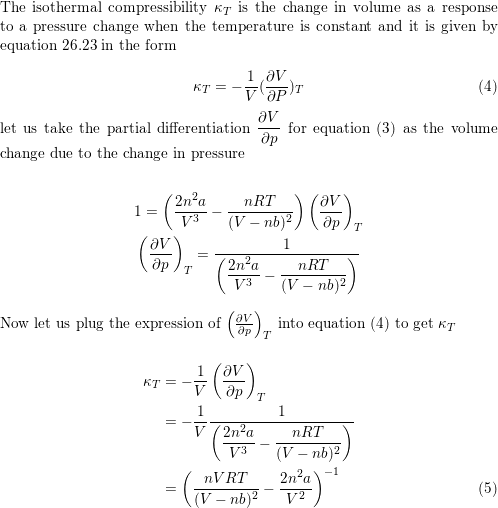
Isothermal compressibility K of an ideal gas is defined as K = 1/V∂ V /∂ P T . nWhat is the isothermal compressibility factor for an ideal gas at 1.0 atm ?

Real Gas Behavior The Compression Factor (Z) [Example #2]
For CO, isotherm is of the type as shown: Near the point A, compr
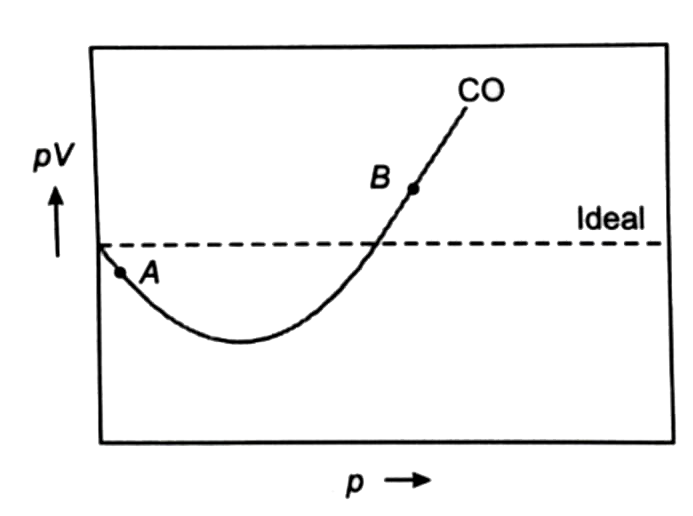
For Co, isotherm is of the type as shown. Near point A, compressibilit