Compressibility factor (Z=(PV)/(nRT)) is plotted against pressure

By A Mystery Man Writer

The given graph represents the variation of Z(compressibility factor =displaystyle frac{mathrm{P}mathrm{V}}{mathrm{n}mathrm{R}mathrm{T}}) versus mathrm{P}, three real gases mathrm{A}, mathrm{B} and C. Identify the only incorrect statement.For the gas C

Telugu] Compressibility factor (Z = (PV)/(nRT)) is plotted against p
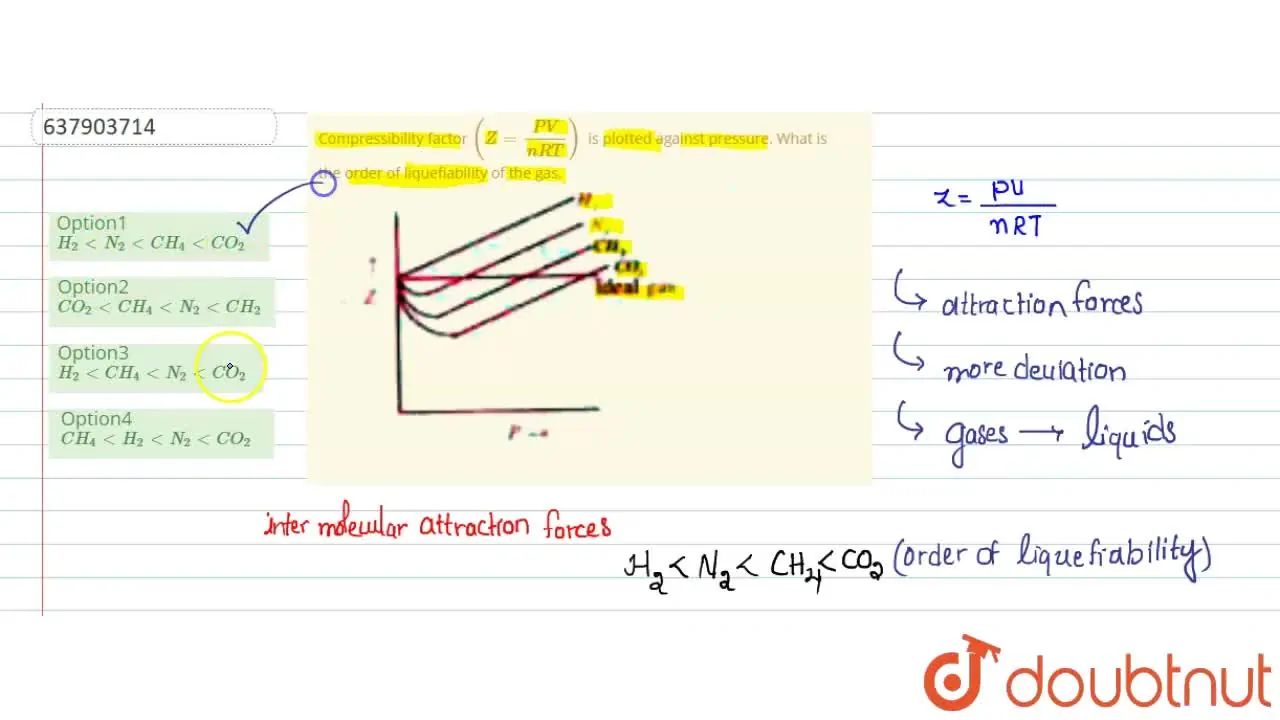
Telugu] Compressibility factor (Z = (PV)/(nRT)) is plotted against p

Determine Compressibility of Gases

The compressibility factor `(Z=PV//nRT)` for `N_(2)` at `223 K` and `81.06 MPa` is `1

Deviation of Real Gases from Ideal Gas Behaviour - Chemistry for ACT PDF Download

PV Compressibility factor Z= nRT is plotted against pressure : N. Ideal gas What is the correct order of liquefiability of the gases shown in the above graph? H

Compressibility factor Z is plotted against pressure p for four different gases A , B , C & D. The correct order of critical temperature of the gasesA. A>B>C>DB. B>A>C>DC. D

REAL GASES, DEVIATION FROM IDEAL GAS BEHAVIOUR

Compressibility factor - Wikipedia

COMPRESSIBILITY FACTOR

Compressibility factor - Wikipedia
- Garota esportiva fitness em roupas esportivas da moda fazendo

- Boob Tape Kit-Boobytape for Breast Lift,14 Pcs Nipple Cover W Petals,36 Pcs Waterproof Body Tape for Sticky Bra

- Redefining Leadership: the 8 Roles Real Leaders Play
- Teen Girls/Womens Bikini Set Color Block Sporty Crop Top High Waisted Cheeky Two Piece Swimsuits : : Clothing, Shoes & Accessories

- Plus Size - Dream Wire-Free Push-Up Bra - Torrid






