The compression factor (compressibility factor) one mole of a van der Waals gas 0°C and 100 atm pressure is found to be 0.5. Assuming that the volume of a gas molecule is

By A Mystery Man Writer
Click here:point_up_2:to get an answer to your question :writing_hand:the compression factor compressibility factor for onemole of a van der waals gas at 0c
Click here👆to get an answer to your question ✍️ The compression factor -compressibility factor- one mole of a van der Waals gas 0-C and 100 atm pressure is found to be 0-5- Assuming that the volume of a gas molecule is negligible- calculate the van der Waals- constant a
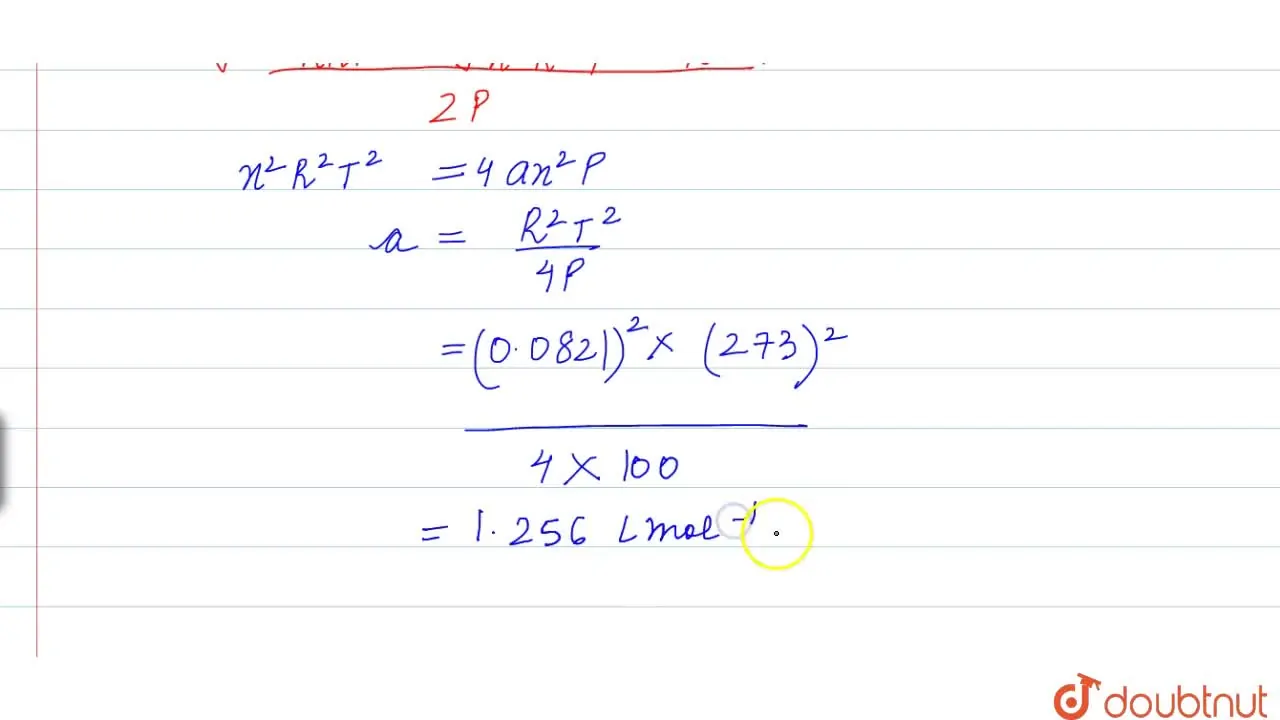
The compressibility factor for definite amount of van der Waals' gas a

1 CHAPTER 6 PROPERTIES OF GASES 6.1 The Ideal Gas

1.7: Connecting the van der Waals and the viral equations: the Boyle temperature - Chemistry LibreTexts

Pick only the incorrect statement.for gas A, a=0,the compressibility factor is linearly dependent on pressure.for gas C,aneq 0,bneq 0,it can be used to calculate a and b by giving lowest P value.for

The compression factor (compressibility factor) for one mole of a vander Waals gas at 0^∘C and
The compressibility factor for one mol of a vanderwalls gas at 0 degree c and 100atm pressure is .5 then what will be the volume of 2 mols of this gas

6.3: Van der Waals and Other Gases - Physics LibreTexts
The compression factor (compressibility factor) for one mole of a van der Waals' gas - Sarthaks eConnect

The compression factor (compressibility factor) for one mole of a Van der..
- Compressibility factor for real gases

- Math cad compressibility factor, z, of real gas using the redlich-kwong equation of state
- Role of Mach Number in Compressible Flows
- the equation of state of a gas is p(v-nb)=rt where b and r are consta - askIITians

- SOLVED: Question: Using the equation for the compressibility factor, Z, calculate the value of Z for water at p = 35 MPa and 500°C (where the constant for steam is R =





