1) 2 23 g ethanol is dissolved in 36 g water. Find mole fraction

By A Mystery Man Writer
Click here:point_up_2:to get an answer to your question :writing_hand:1 223 g ethanol is dissolved in 36 g water find mole fraction of ethanol2
Click here👆to get an answer to your question ✍️ -1- 2 23 g ethanol is dissolved in 36 g water- Find mole fraction of ethanol -2- 0-5 -3- 0-2 -4- 0-8 TIN

36g water and 828g ethyl alcohol form an ideal solution. The mole

Development of a reliable empirical correlation to calculate
In a certain solution of ethanol and water, the mole of fraction
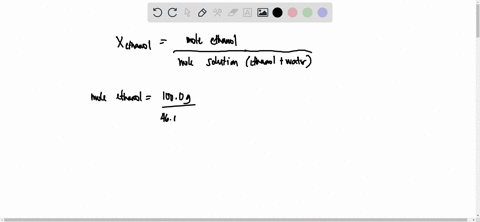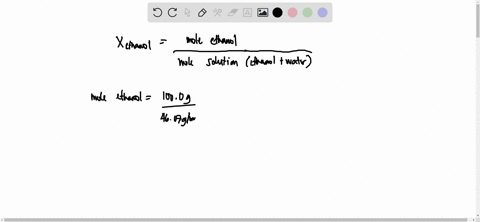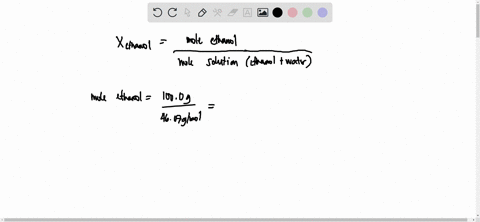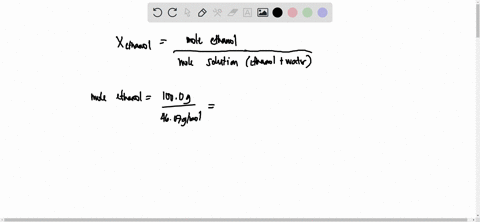
SOLVED: 23 gram ethanol is dissolved in 36 gram water. Find mole

23 g ethanol is dissolved in 36 g water. Find mole fraction of

Ethanol - Wikipedia

Deptt. Of Applied Sciences Govt. Polytechnic College For Girls

23 g ethanol is dissolved in 36 g water. Find mole fraction of

Calculate the molarity of a solution of ethanol in water in which

23 gram of ethyl alcohol is dissolved in 54 gram of water

22 ethanol is dissolved in 36 g water. Find mole fraction of

Calculate the mole fraction of water in a mixture of 15 g water
What is the molarity of a solution when 45g of glucose is present
- Bralettes Red Lace Bras Victoria's Secret Ireland

- How 'Citizen Kane' gave The White Stripes one of their best songs

- Floral Lace Bra Panties Solid Push Bra Semi sheer Thongs - Temu

- Lululemon Wunder Under High-Rise Tight 31 in Luon Variegated Knit Black - SZ 6

- WOMEN'S NIKE PRO TIGHTS - Nike - WOMEN'S - CLOTHING - BADMINTON