32. 80 g of h2 is reacted with 80 g of o2 to form water. find out the mass of water obtained.which substance is the limiting reagent.
By A Mystery Man Writer
32. 80 g of h2 is reacted with 80 g of o2 to form water. find out the mass of water obtained.which substance is the limiting reagent.
32- 80 g of h2 is reacted with 80 g of o2 to form water- find out the mass of water obtained-which substance is the limiting reagent

52. 80 g of H, is reacted with 80 g of O, to form water. Find out the mass of water obtained. Which substance is the limiting reagent?
In the combustion of butane, how many grams of excess water will you have with a reaction between 20.0g of butane and 20.0g of oxygen? - Quora

80 g of H_(2) is reacted with 80 g of O_(2) to form water. Find out the mass of water obtained.
media.springer/m685/springer-static/imag

Answered: 3. Hydrogen and oxygen gas combine to…
How many grams of water can be produced the combination of 8 grams of oxygen and 8 grams of hydrogen?

Interface, Vol. 32, No. 2, Summer 2023 by The Electrochemical Society - Issuu

PS GR 11 Session 11 LN
Hibbitts Group Publications

In the reaction H2 + O2 =H20. If 6g of H, combines with 64g of Oz. Find mass of Excess reagent left? 32 g 48 g 16 g None of these

Hydrogen and oxygen combine in the ratio of 1:8 by mass to form water
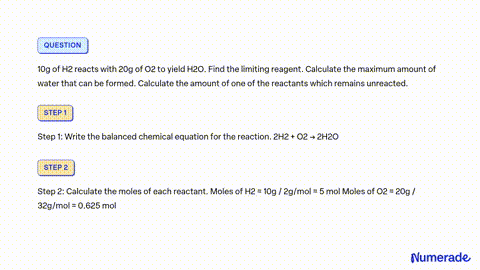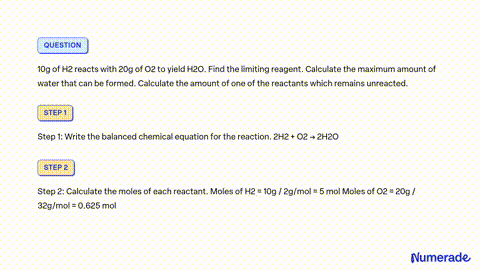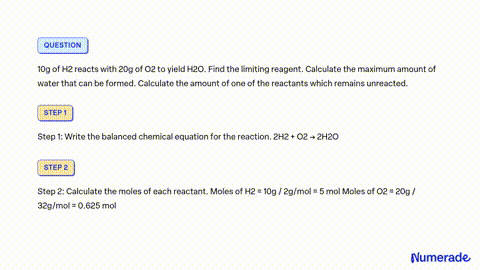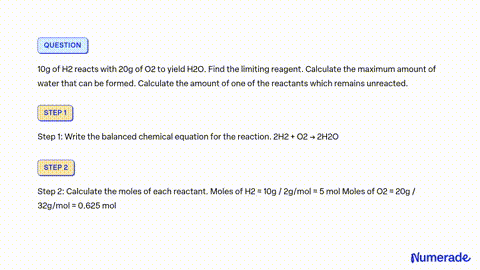
SOLVED: 80 g of H2 is reacted with 80 g of O2 to form water. Find out the mass of water obtained. Which substance is the limiting reagent?

80 g of `H_(2)` is reacted with 80 g of `O_(2)` to form water. Find out the mass of
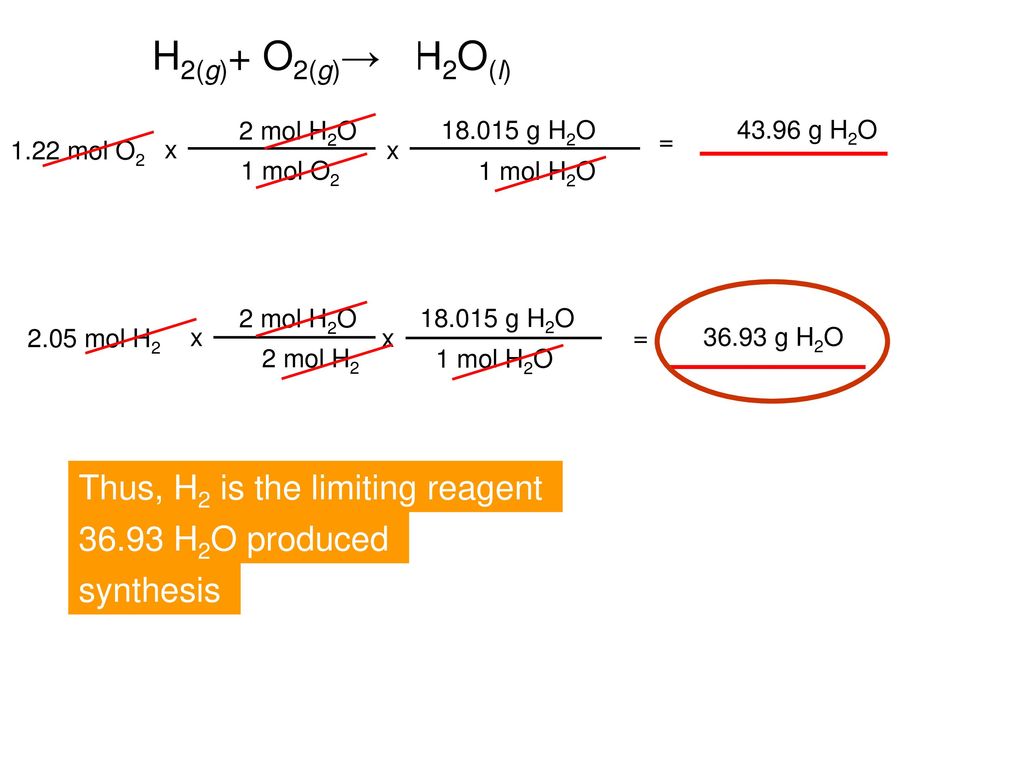
2H2(g)+ O2(g)→ 2H2O(l) Thus, H2 is the limiting reagent - ppt download

52. 80 g of H, is reacted with 80 g of O, to form water. Find out the mass of water obtained. Which substance is the limiting reagent?
- Quest Tortilla Style Protein Chips Nacho Cheese, 32 g

- Cubot J10, smartphone, Android 11, tela de 4 polegadas, telefones celulares MINI, 32 GB de RAM, Dual SIM 3G, identificação facial, bateria de 2350 mAh, câmera traseira de 5 MP, celular barato com frete grátis

- Console de vídeo Game 2.4G, 4K, com dois controles sem fio. Possui

- Pepero Palitinhos c/ Chocolate Choco Cookie 32g - HARU PRODUTOS

- Fralda Looping Looney Tunes Mega G 32 Unidades

- Jogger Mujer XPOWER - Ref 7083 – xpower colombia

- GoFit Pattern Yoga Mat w/ Yoga Pose Wall Chart, 3.5mm, 24 X 68

- Selfcare Set Of 2 Seamless Moulded Cup Bras-White at Rs 360

- 2 Wide Spiked Studded Leather Dog Collar for Medium Large Dogs Pit Bull Mastiff

- Wide Hole Dome Lid, PET 98mm Diameter Plastic Cold Cup Lids [Case of 1000 Counts]
