The entropy change for the conversion of 36 g water to vapour at
By A Mystery Man Writer
The entropy change for the conversion of 36 g water to vapour at its boiling point at 1 atm is (Enthalpy of vaporization for water is 40.63 kJ mol–1)
The entropy change for the conversion of 36 g water to vapour at its boiling point at 1 atm is -Enthalpy of vaporization for water is 40-63 kJ mol-1

Calculate the entropy change when `3.6g` of liquid water is completely converted

calculate the entropy change for the conversion of 2moles of liquid water at 373 Kelvin to vapours, if
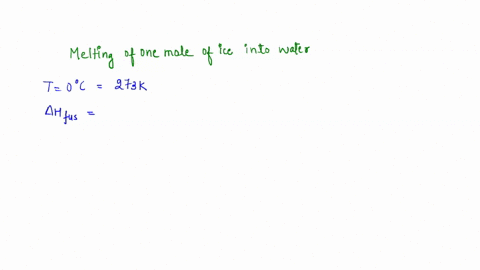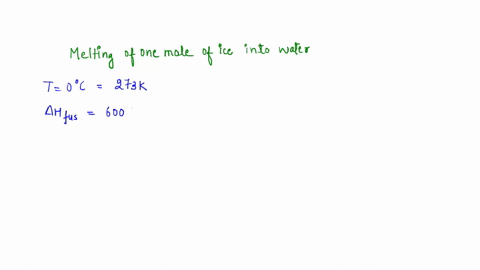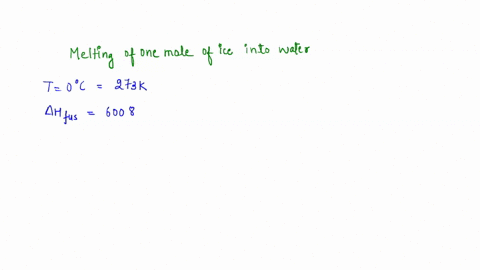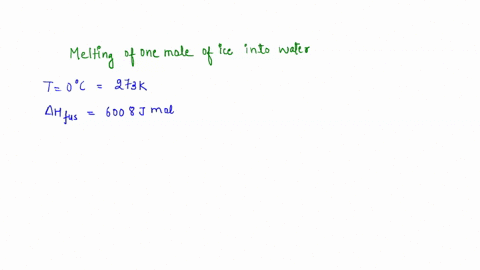
⏩SOLVED:Calculate the entropy change for the conversion of…

The entropy change associated with the conversion of 1 kg of ice at 273 K to water vapours at 38

If water vapor is assumed to be a perfect gas, molar enthalpy change for vaporization of 1 mol of

The Second Law of Thermodynamics - University Science Books

66. The entropy change for the conversion of 36 g of water to vapour at 100°C (Normal boiling point) is

Q The enthalpy of vap of Coto is 30.8 kJ/mol its bp. 480.1°C). Calculate the Entropy change in going from ! Liquid to vapour My Vapour to liquid, 80.1°C

Isotonic separation enabled efficient low-grade heat conversion with thermal-responsive ionic liquids - ScienceDirect

Phase transition - Wikipedia

Calculate the entropy change in surroundings when 1.00 mol of H2O (l) is formed under standard.

3) 6025 JAK (4) 602.5 JIK 87. Calculate the entropy change the conversion of 36 g water to vapour 373 K; AH, HO=40.63 k mor! (2) 202.07 JAK () 602 JK (4)

The entropy change when 36g of water evaporates at 373 K is :- (DeltaH

Hybrid solar evaporation system for water and electricity co-generation: Comprehensive utilization of solar and water energy - ScienceDirect
- Dia 100 Cura Diarreia Pasta 36 G Real H

- Buy Cadbury Dairy Milk Crackle Chocolate Bar 36 Gm Online At Best

- Whey Pro 36g de Proteina Body Nutry Pacote 900g - Ganho de Massa

- Buy Cadbury Dairy Milk Fruit Nut Chocolate Bar 36 Gm Online At Best Price of Rs 45 - bigbasket

- Protégé 36 Drop-Bottom Rolling Polyester Travel Duffel - Blue with Black


)


