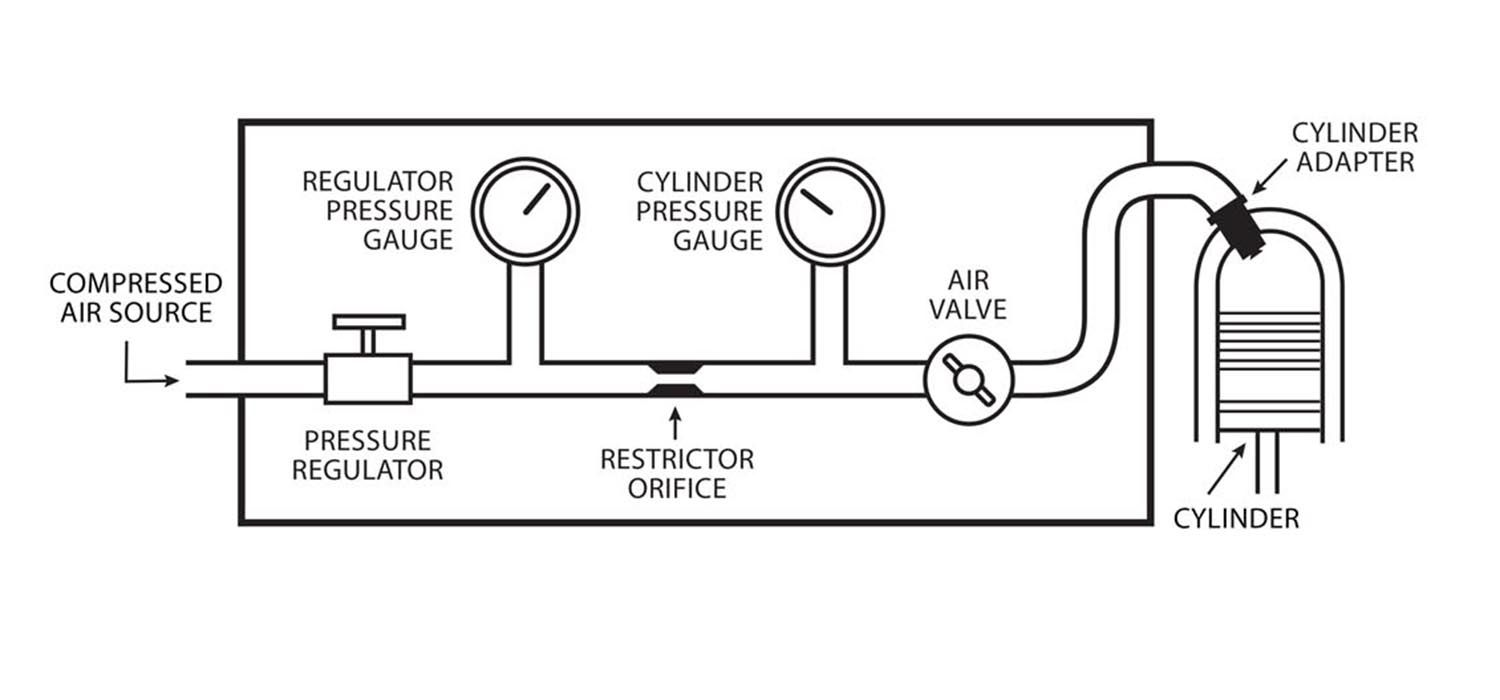
Compression of a gas due to external pressure and the

By A Mystery Man Writer
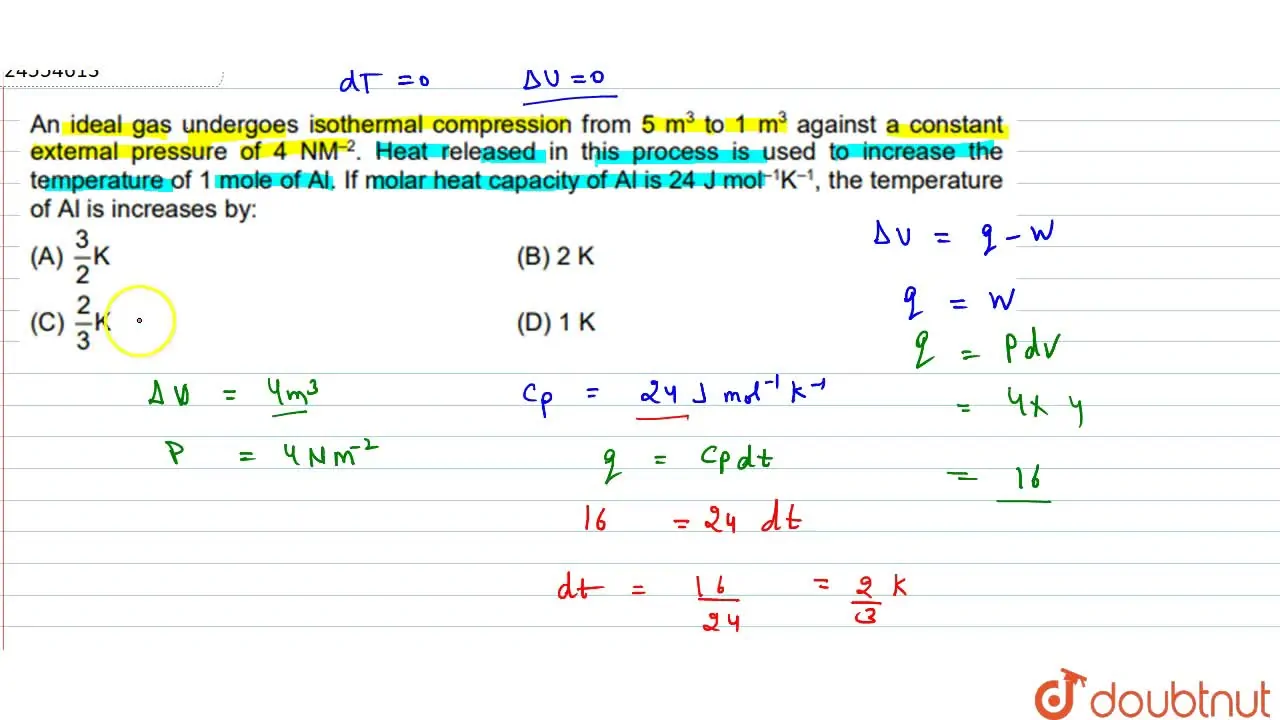
An ideal gas undergoes isothermal compression from 5 m^(3) to 1 m^(3)

An ideal gas undergoes adiabatic expansion against constant external pressure. Which of

The gas mixture inside one of the cylinders of an automobile
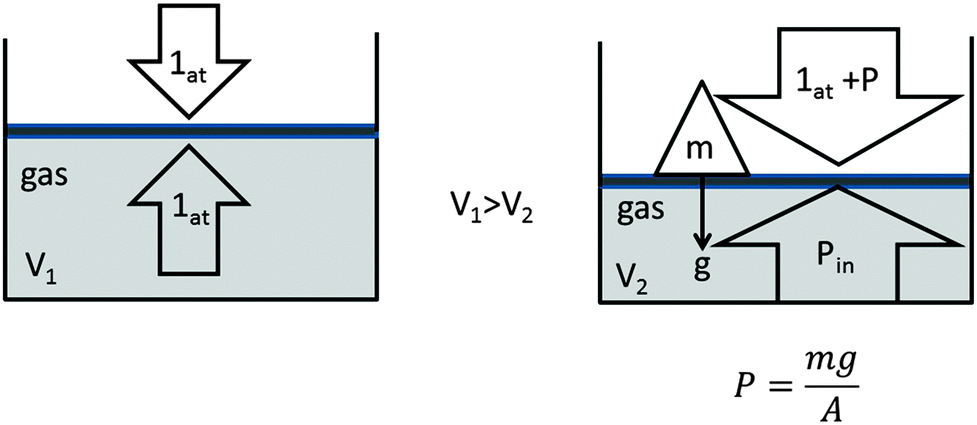
Natural laws and ontological reflections: the textual and didactic

thermodynamics - Are you supposed to use the internal or external pressure for the $pV$ work integral? - Physics Stack Exchange
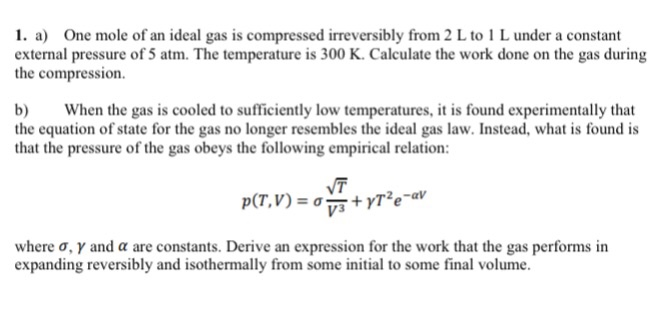
Solved 1. a) One mole of an ideal gas is compressed

Q. 2 mole of an ideal gas undergoes isothermal compression along three different paths (i) reversible compression from P; = 2 bar and V; = 4 L to P, = 20 bar (

One mole of an ideal gas is compressed from 500 cm^(3) against a

A piston having 0.033 mol of gas at 35.0 C expands from 0.77 L to 2.00 L. Calculate the work performed if the expansion occurs (a) against an external pressure of 0.455
Solved A gas is compressed from an initial volume of 5.35 L

Waldo QUIROZ, Professor (Full), PhD Chemistry
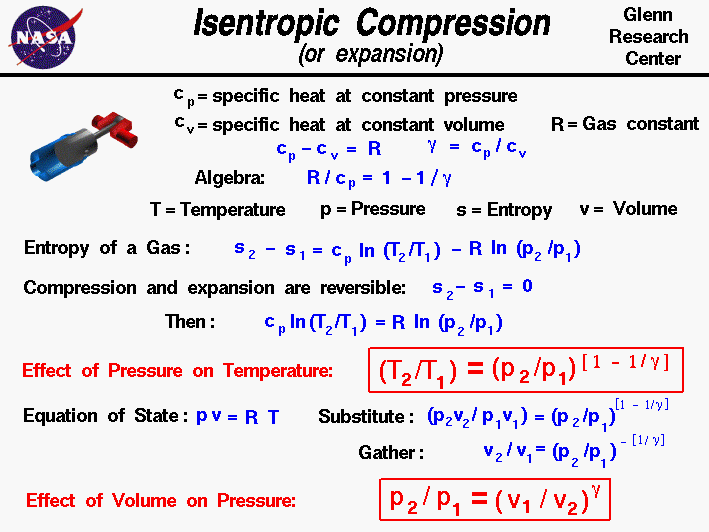
Isentropic Compression or Expansion
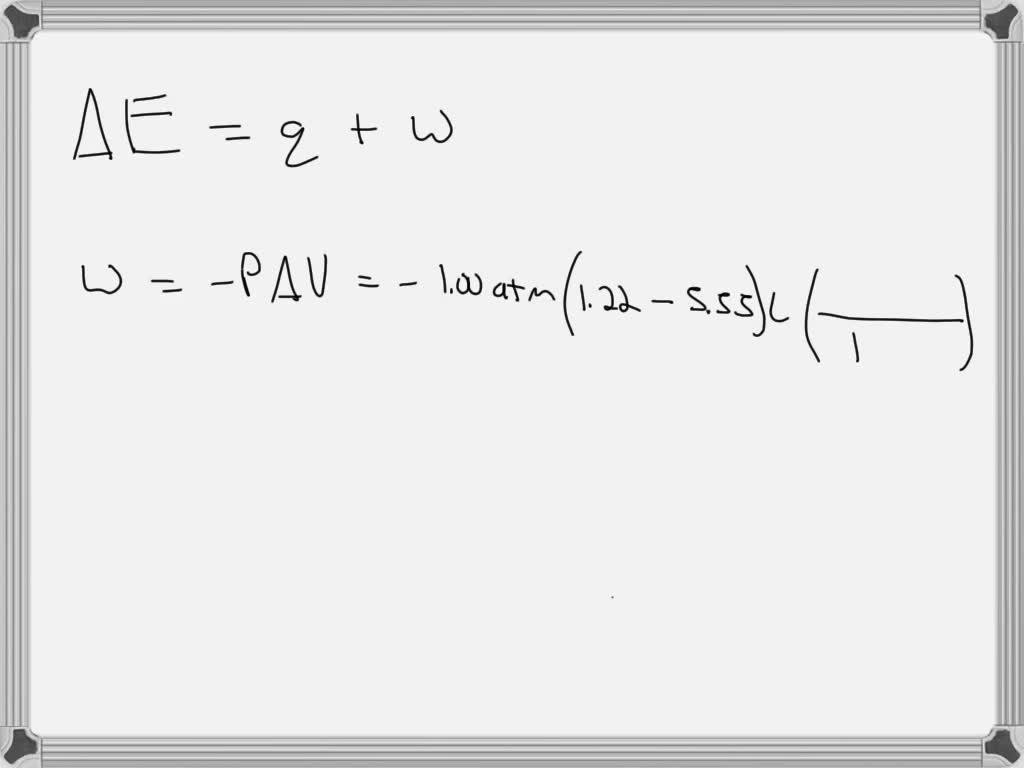
SOLVED: A gas is compressed from an initial volume of 5.55 L to a final volume of 1.22 L by an external pressure of 1.00 atm. During the compression the gas releases

Compression Pressure - an overview
- Buy Bodycare B-C-D Cup Bra In Skin-White Color (Pack of 2) - 34C

- Spaghetti Strap Beaded Lace Fit And Flare Wedding Dress With Open

- Nancy Bolen City Girl Womens Silk Shirt Large Blue Pink Tab Sleeve Button Up

- Tripp Chain To Chain Pants [Black/Red] – VampireFreaks

- Women Casual High Waist Pants Ladies Summer Baggy Loungewear Trousers Loose Fit