At 300 K, 36 g of glucose present per litre in its solution has an osm

By A Mystery Man Writer
pi=CRT" (C = molar concentration)" (pi(1))/(pi(2))=(C(1))/(C(2))," "(4.98)/(1.52)=(36//180)/(C(2))" or "C(2)=(36)/(180)xx(1.52)/(4.98)="0.061 M"

At 300K, 26g of glucose present in a litre of its solution has an osmotic pressure of 4.98 bar.

S2 Physiology Unit 2 - Body Fluid Physiology Flashcards

Heptane and octane form ideal solution. At 373 K, the vapour pressures
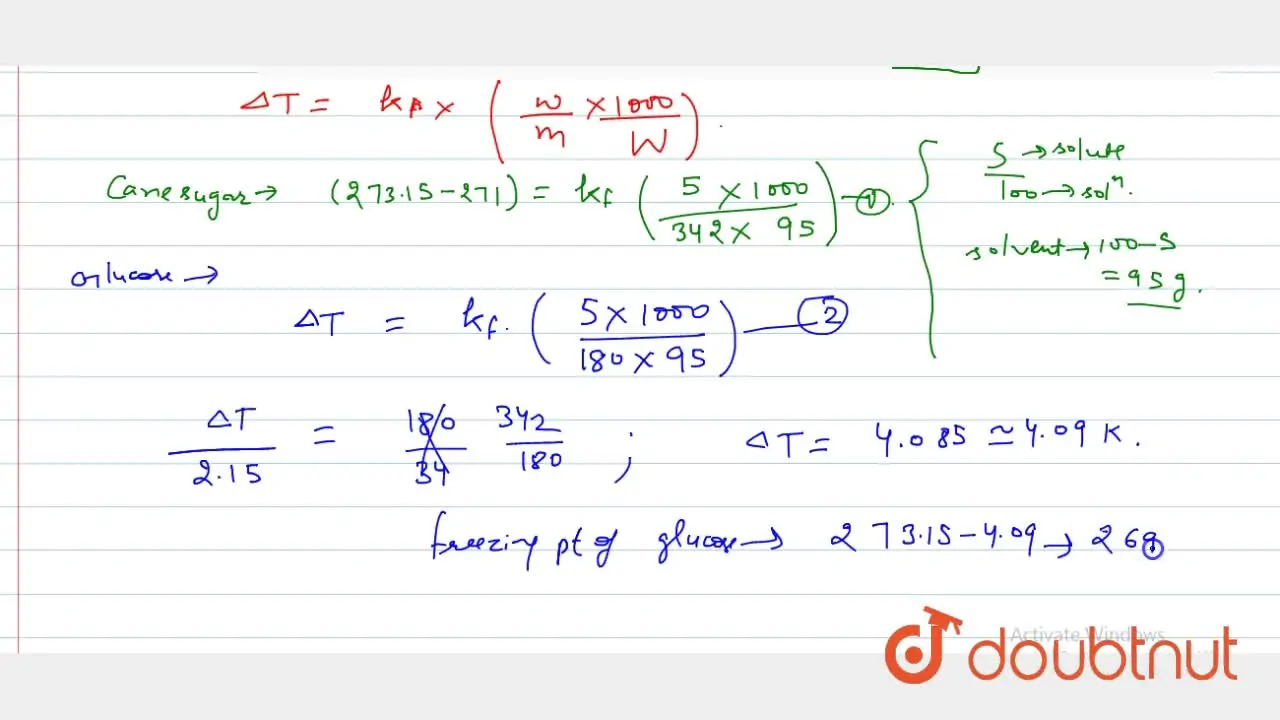
A 5% solution (by mass) of cane sugar in water has freezing point of 2
The current carrying ions are not necessarily discharged at the electr
PDF) Volume kinetics of glucose solutions given by intravenous infusion

The osmotic pressure of blood is 8.21 atm at 37^(@)C. How much glucose

20 g of solute was added to 100 g of water at 25^(@)C. The vapour pres

12 At 300 K, 36 g of glucose present in a litre of its solution has an osmotic pressur of 4.98 bar. If the osmotic pressure of the solution is 1.52 bars

At 25^(@)C, the vapour pressure of pure water is 23.76 mm of Hg and th

BIL360 DuBois Chapter 5: Transport of Solutes and Water Flashcards

Hyaluronic Acid: The Influence of Molecular Weight on Structural, Physical, Physico-Chemical, and Degradable Properties of Biopolymer. - Abstract - Europe PMC

Calculate the osmotic pressure of a solution containing 17.1 g of cane
- High Waisted Waist Trainer Shapewear Body Tummy Shaper Fake Ass Butt Lifter Booties Hip Pads Enhancer at Rs 3161, Koramangala, Bengaluru

- Trinny Woodall takes her daughter, Lyla, to a red carpet event
- NEW OLD NAVY LEGGING TRY ON REVIEW / EXTRA HIGH WAISTED POWERCHILL

- Buy Bonds Girls Underwear Briefs Yellow And White Striped Everyday Kids Undies - MyDeal

- The test vehicle, a John Deere Gator holding multiple sensors of which






