The compressiblity factor a gas obeying van der Waals' equation of state is given by V V-b RTV (2) a ✓ RTV V-b V-b RTV (3) Va (4) RTV V-6

By A Mystery Man Writer
Click here:point_up_2:to get an answer to your question :writing_hand:the compressiblity factor for a gas obeying vander waals equation of state is given byvvbrtv2
Click here👆to get an answer to your question ✍️ The compressiblity factor a gas obeying van der Waals- equation of state is given by V V-b RTV -2- a - RTV V-b V-b RTV -3- Va -4- RTV V-6

At a high pressure, the compressibility factor (Z) of a real gas is us
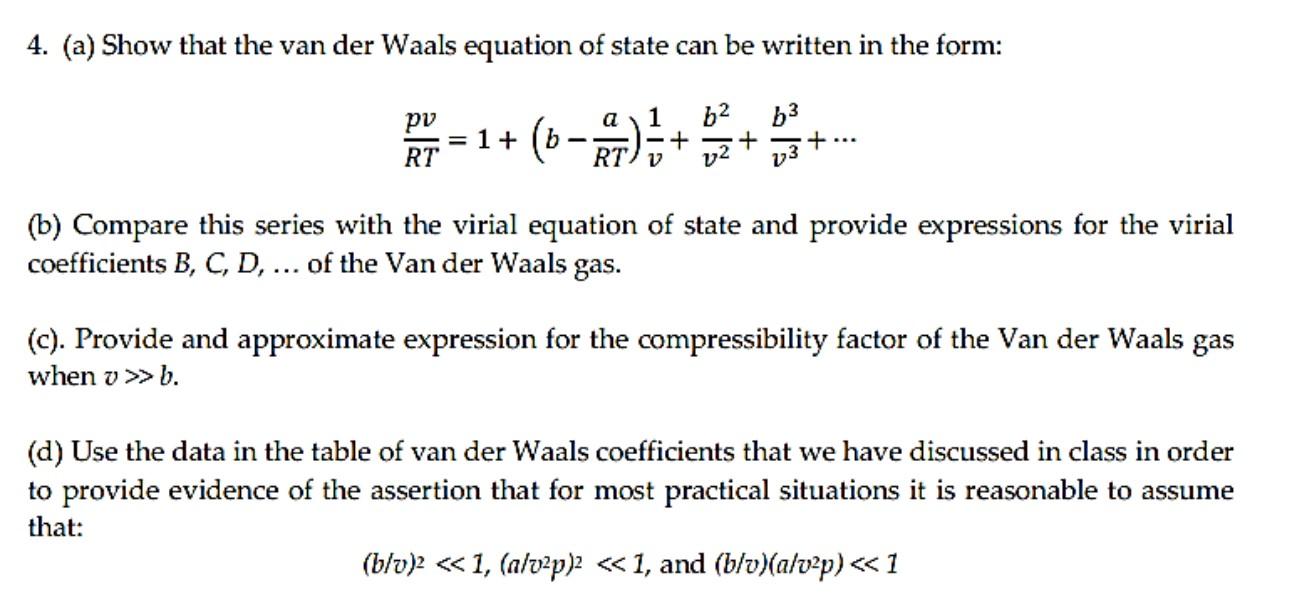
Solved 4. (a) Show that the van der Waals equation of state
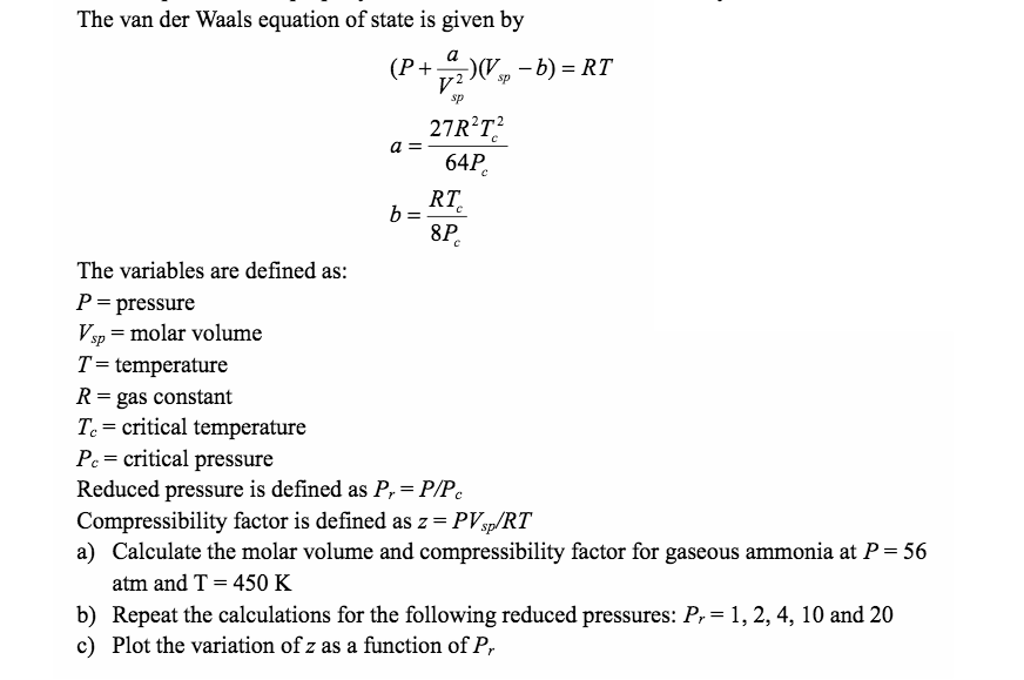
The van der Waals equation of state is given by (P +
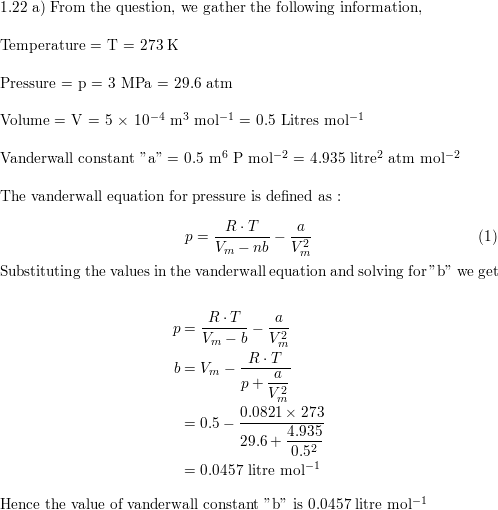
a) A certain gas obeys the van der Waals equation with $a =

Compressibility factor (Z) for a van der Waals real gas at critical point is
The compression factor (compressibility factor) for one mole of a van der Waals' gas - Sarthaks eConnect

Theory of gases - Compressibility factor according to van der Waals equation

If Z is a compressibility factor, van der Waals equation at low pressure ..

Solved For a gas obeys the van der Waals equation of state P

Elementary General Thermodynamics - Martin V. Sussman PDF, PDF, Entropy

Assertion :Compressibility factor Z according to van der Waal's equation may be written as Z=cfrac {1}{1-(cfrac {nb}{V})}-cfrac {an}{RTV}. Reason: For real gases Z > < 1.Both Assertion and Reason are correct and
- ChemE 260 Equations of State April 4, 2005 Dr. William Baratuci Senior Lecturer Chemical Engineering Department University of Washington TCD 2: E & F CB. - ppt download

- If Z is a compressibility factor, van der Waals equation at low pressure ..

- Slope of graph of compressibility factor(Z) with pressure(P) for hydrogen gas at any pressure i
- PPT - GASES PowerPoint Presentation, free download - ID:2088317

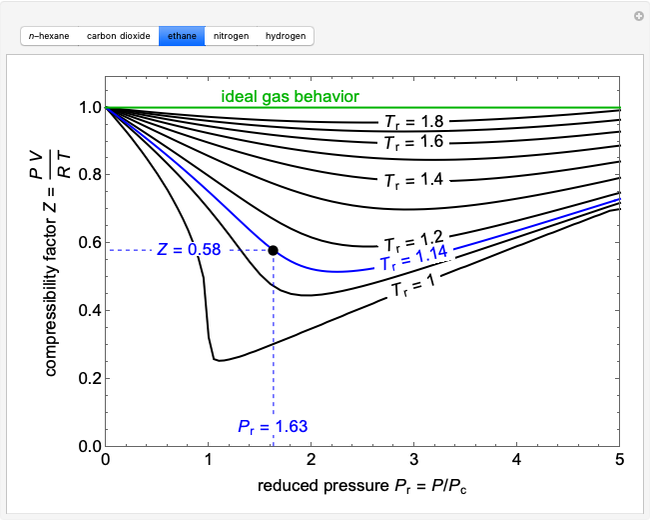
- Compressibility Factor Charts - Wolfram Demonstrations Project