plotting - How to plot Compressibility factor Z vs Pressure P

By A Mystery Man Writer

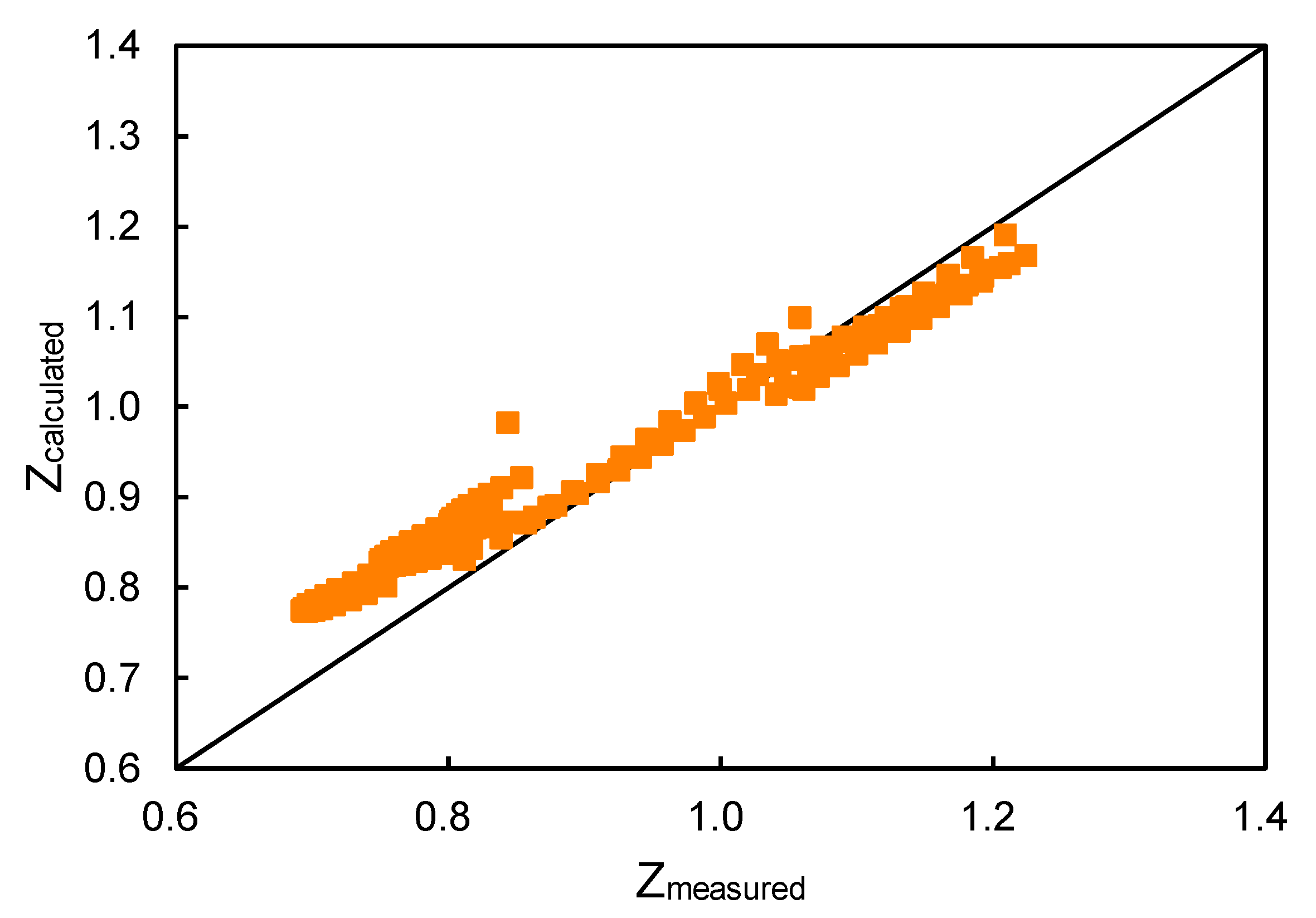
Simple Equation Real Gas Compressibility Factor Z
Is z (compressibility factor) vs P (pressure) graph drawn by changing volume? If it is why it isn't drawn by changing mole - Quora

As the pressure approaching zero i.e., very low pressure, the curves plotted between compressibility factor Z and P n mole of gases have the following characteristics.I. The intercept on the y-axis leads

Material Balance Analysis Theory

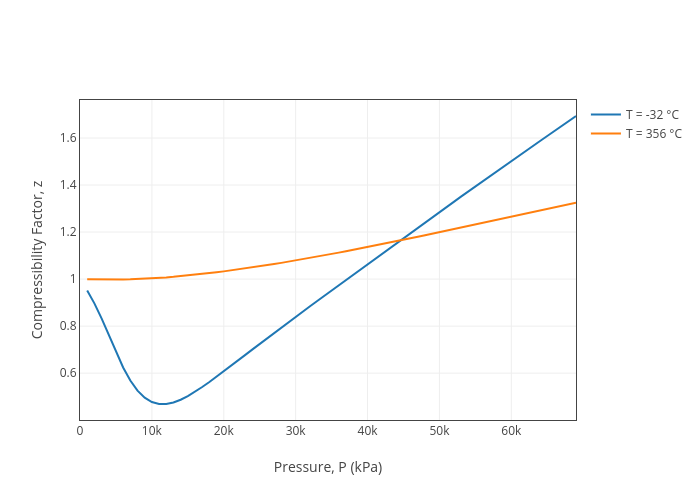
Improved description of the liquid phase properties of Methane: density, enthalpy, plus saturated vapor compressibility factor
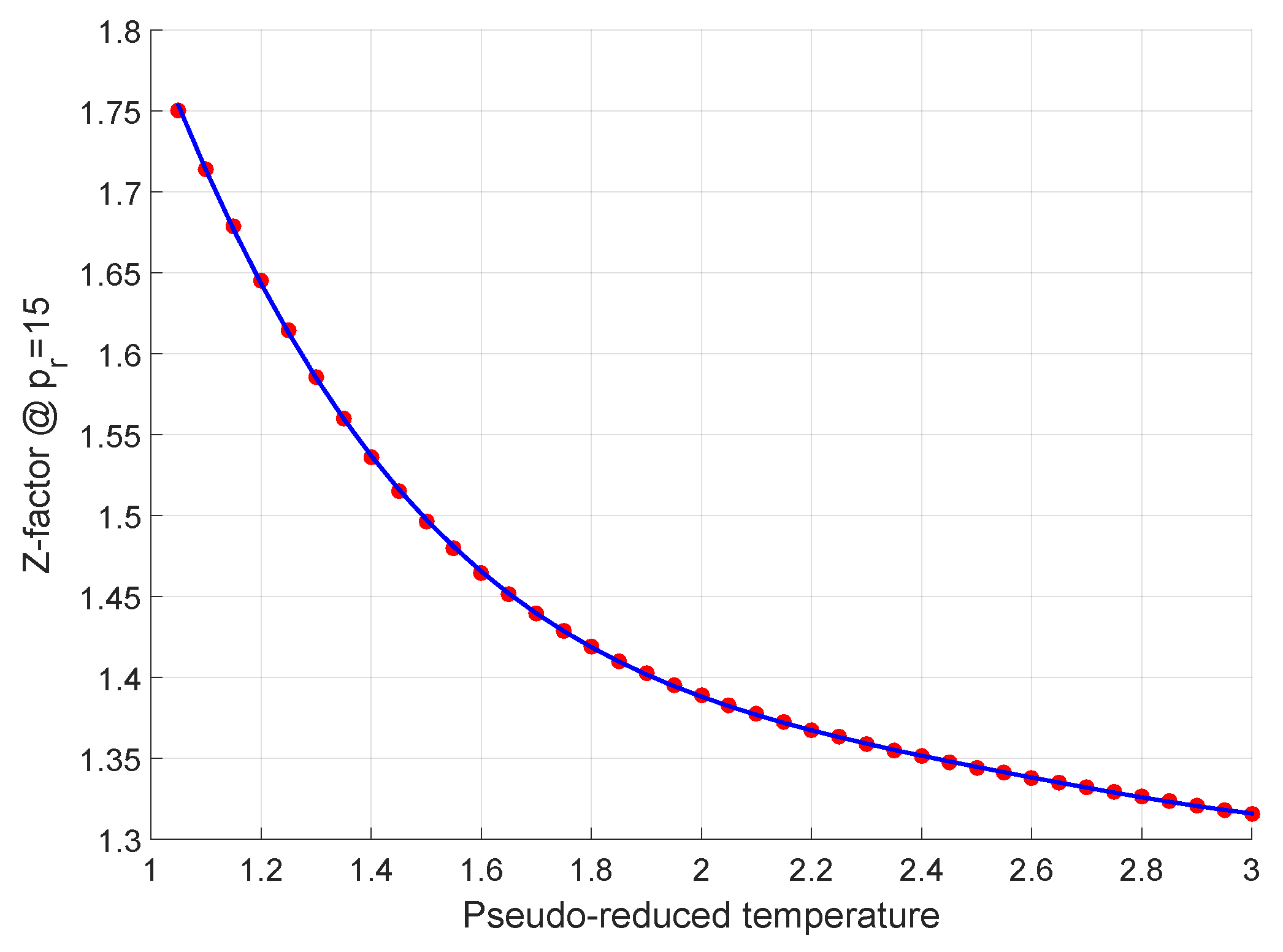
Energies, Free Full-Text

The graph of compressibility factor (Z) vs. P for one mole of a real gas is shown in following
A plot of volume (V ) versus temperature (T ) for a gas at constant pressure is a straight line passing through the origin. - Sarthaks eConnect

A real gas M behaves almost like an ideal gas. Graph 1 is obtained by plotting volume, V against temperature, T for x mol of gas M at pressure, P_1. a. Suggest

Consider the graph between compressibility factor Z and pressure P The correct increasing order of ease of liquefaction of the gases shown in the above graph is
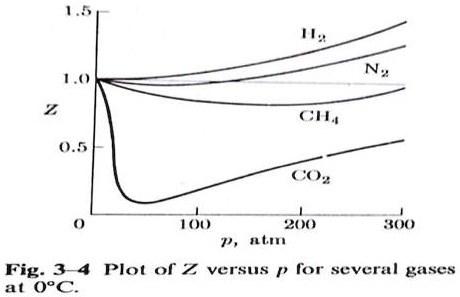
SOLVED: Subject: Compressibility Factor What is the analysis of the graph? 1.5 N 1.0 Z CHA 0.5 CO2 1 0 100 200 P, atm 300 Fig. 3-4: Plot of Z versus p for several gases at oc.

The given graph represent the variations of Z (compressibility factor (Z)=dfrac {pV}{nRT}) versus P, three real gases A, B and C. Identify the only incorrect statement.For the gas B, b=0 and its
- WOMENS BONDS FULL Brief Satin Touch Cottontails High Waist

- Short Sleeve Puffy Wedding Dress, Wedding Short Puff Sleeves Dress

- HICCVAL Mujer 2 Piezas De Algodón De Lino Conjuntos 2023 Casual 3/4 Manga Tops Y Pantalones Largos De Pierna Ancha Sweatpants Set Loungewear Light khaki-4XL : : Ropa, Zapatos y Accesorios

- Rekucci Womens Ease Into Comfort Fit Barely Bootcut Stretch Pants

- PALMA - YANES SPARKLE – Triangl