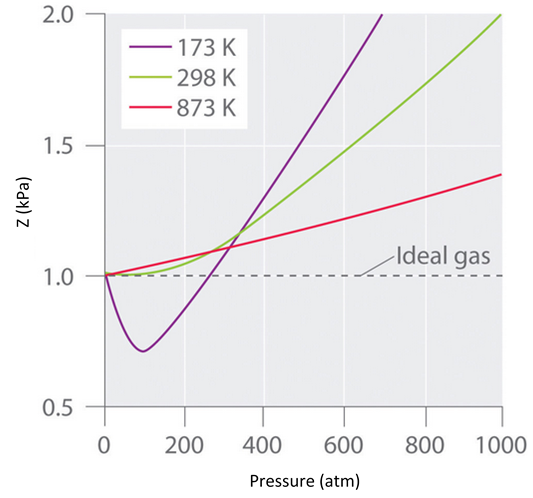
In the following compressibility factor (Z) vs. pressure graph 300 K, the compressibility of CH_{4} pressure < 200 bar deviates from ideal behaviour becauseThe molar volume of CH_{4} is than its molar

By A Mystery Man Writer
Click here:point_up_2:to get an answer to your question :writing_hand:in the following compressibility factor z vs pressure graph at 300 k the compressibility of
Click here👆to get an answer to your question ✍️ In the following compressibility factor -Z- vs- pressure graph 300 K- the compressibility of CH-4- pressure - 200 bar deviates from ideal behaviour becauseThe molar volume of CH-4- is than its molar volume in the ideal stateThe molar volume of CH-4- is than its molar volume in the ideal stateThe molar volume of CH-4- is same as that in its ideal stateIntermolecular interactions between CH-4- molecules decreases
4.2: Real Gases (Deviations From Ideal Behavior) - Chemistry LibreTexts

NCERT Exemplar Class 11 Chemistry Solutions Chapter 5 - States of Matter: Gases and Liquids

Compressibility Chart - an overview

The following graph is plotted between compressibility factor Z versus pressure of a gas at different temperatures.Which of the following statements is /are correct?

Compressibility factor - Wikipedia

gas laws - Compressible Factor - Chemistry Stack Exchange

Reading Compressibility Factor Charts

Non-Ideal Gas Behavior Chemistry: Atoms First
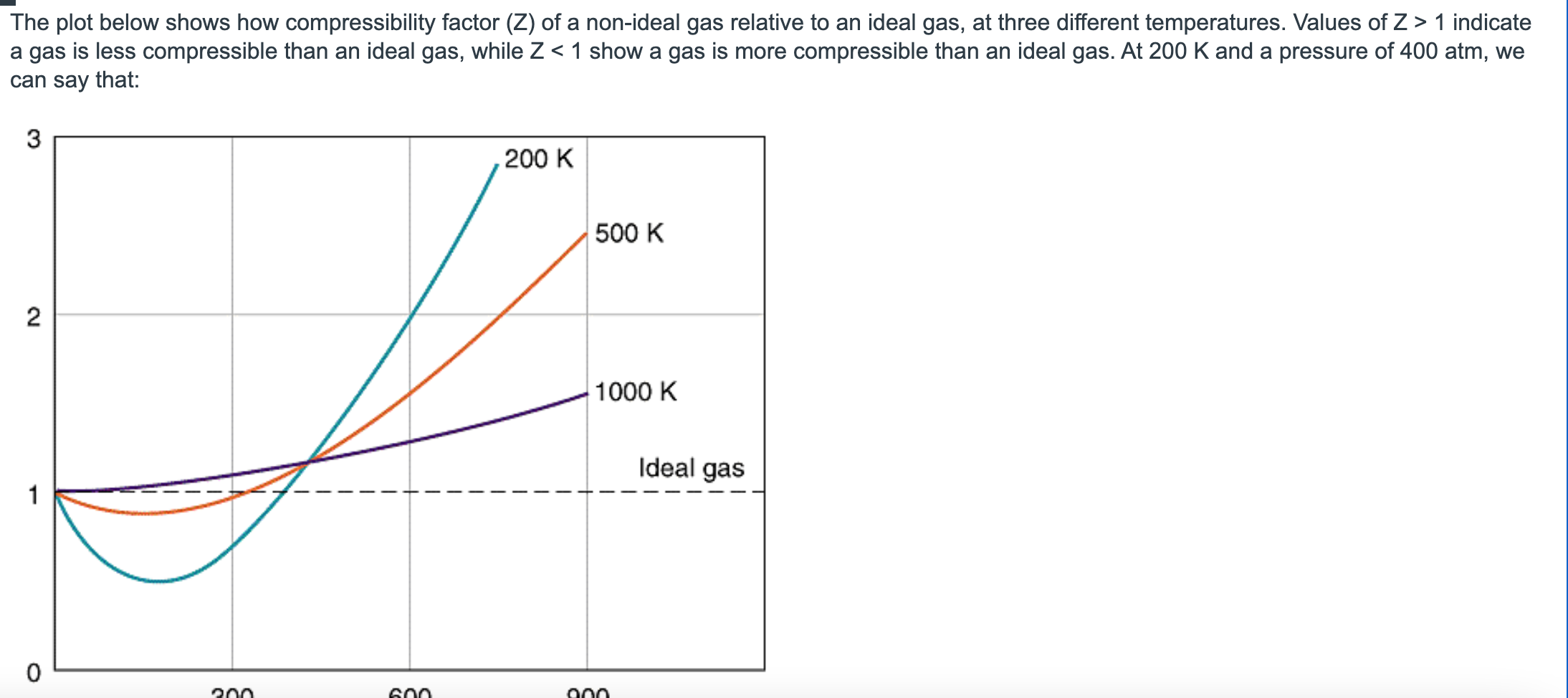
Solved The plot below shows how compressibility factor (Z)
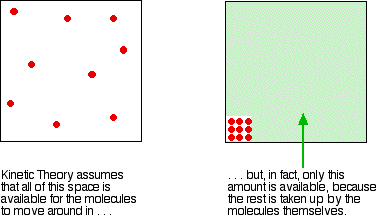
Real gases

The graph of compressibility factor (Z) v/s P 1 mol of a real gas is shown in following diagram. The graph is plotted 273 K temperature. If slope of graph very high
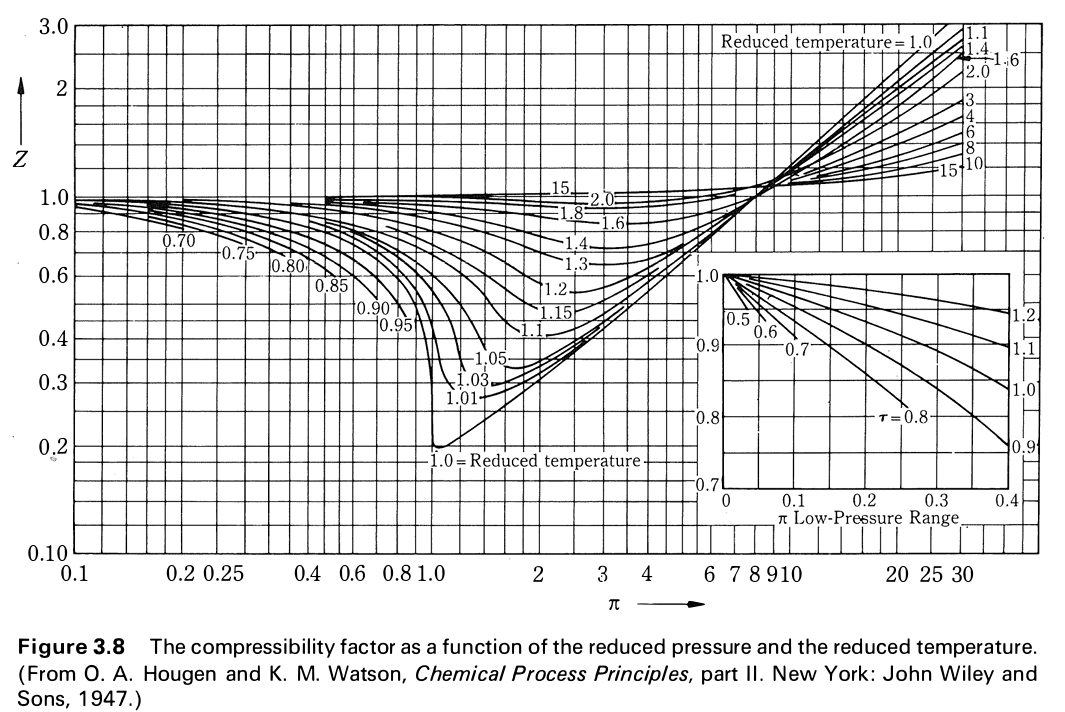
Solved Use the graph of compressibility factors in terms of

Non-Ideal Gas Behavior Chemistry: Atoms First
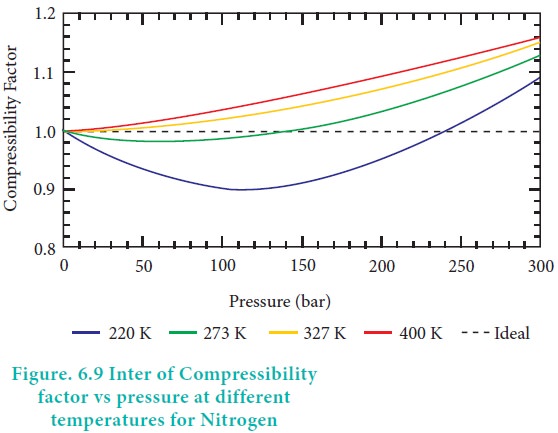
Compressibility factor Z - Gaseous State

Compressibility Factor of Gas, Overview, Equation & Chart - Lesson
- Solved The plot below shows how compressibility factor (Z)
- Compressibility factor Z = PV / nRT is plotted against pressure as shown below:What is the correct order for the liquefiability of the gases shown in the above graph? A. CO 2
- Graph of Compressibility Factor (Z) versus Pressure (Atm)

- physical chemistry - Why do some gases have lower value of Z for a particular pressure? - Chemistry Stack Exchange

- My publications - CHM 201-LECTURE IV-REAL GASES - Page 8 - Created with Publitas.com

- Nike Neon Orange Ribbed Sports Bra

- Girls Training Bra LOT OF 2 SPORTS BRALETTE Pullover GRAY PINK Size S / 30

- Drawstring Shorts
- Come join the ultimate sub-reddit dedicated to vintage Victoria's Secret panties! : r/Vintage_VS_Panties

- Food For The Mat: Model Behavior Crazy yoga poses, Partner yoga poses, Yoga poses advanced



