The FDA's rule change requiring providers to inform women about

By A Mystery Man Writer
The FDA's rule change requiring providers to inform women about breast density could lead to a flurry of questions
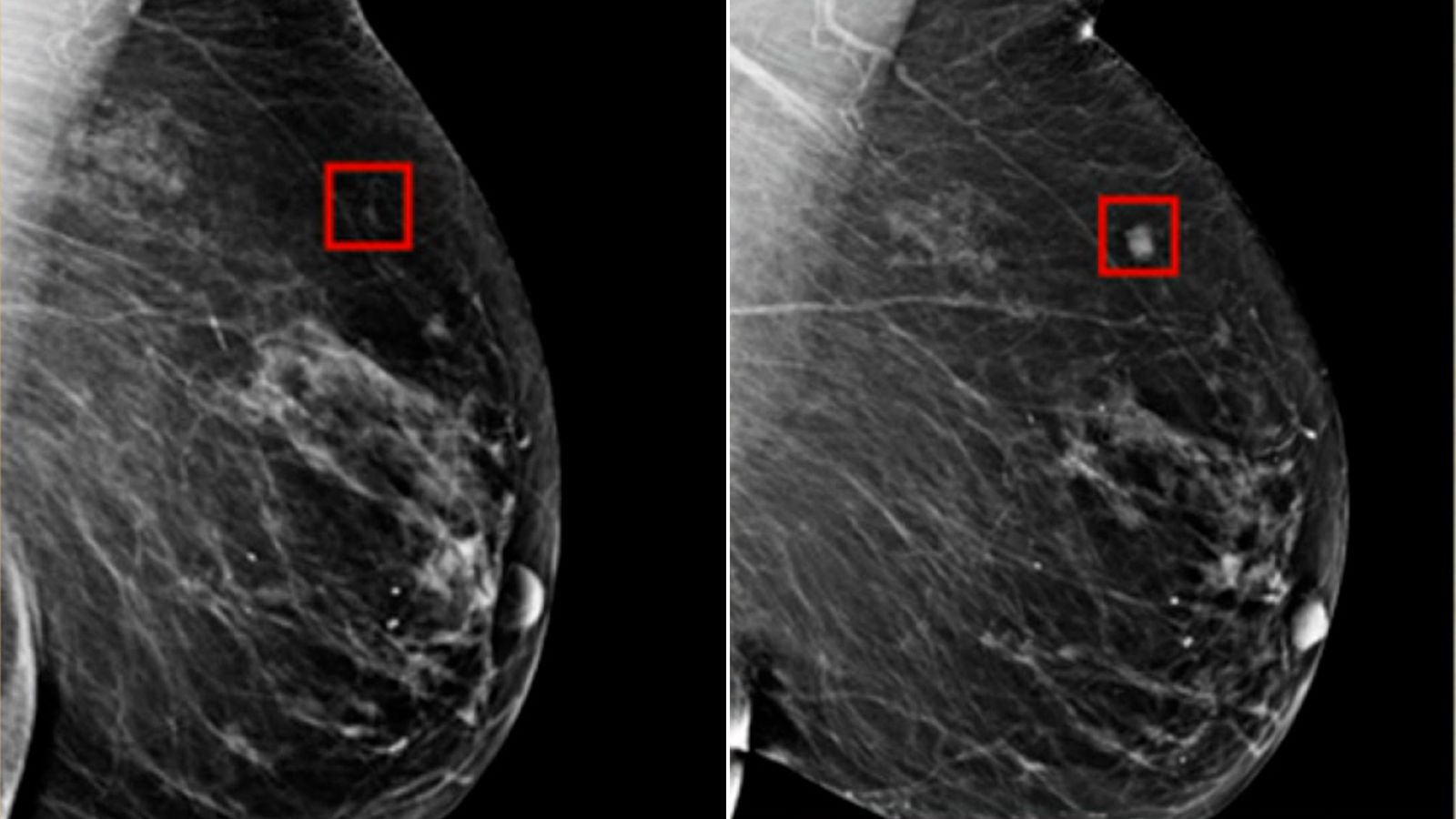
FDA to require mammogram reports include breast density information

Daniel (@lehrerdan1) / X

It's not how much we've done, but how much we are doing!' - UPMC

Medical practitioners will have to notify patients about breast density in mammograms under new FDA regulations - CBS News

FDA to Implement New Mammogram Regulations to Support Women with Dense Breasts
FDA says mammogram facilities must notify women if they have dense breast tissue - ABC News

Women's Healthcare, A Clinical Journal for NPs on LinkedIn: The

Breast cancer screening – News, Research and Analysis – The

The medical research gender gap: how excluding women from clinical trials is hurting our health, Health & wellbeing

FDA Resumes Move to Regulate LDTs, Likely Setting up Legal Battle With Lab Industry

Retail pharmacies can now offer abortion pill, FDA says - POLITICO

Be inforMD: know your breasts
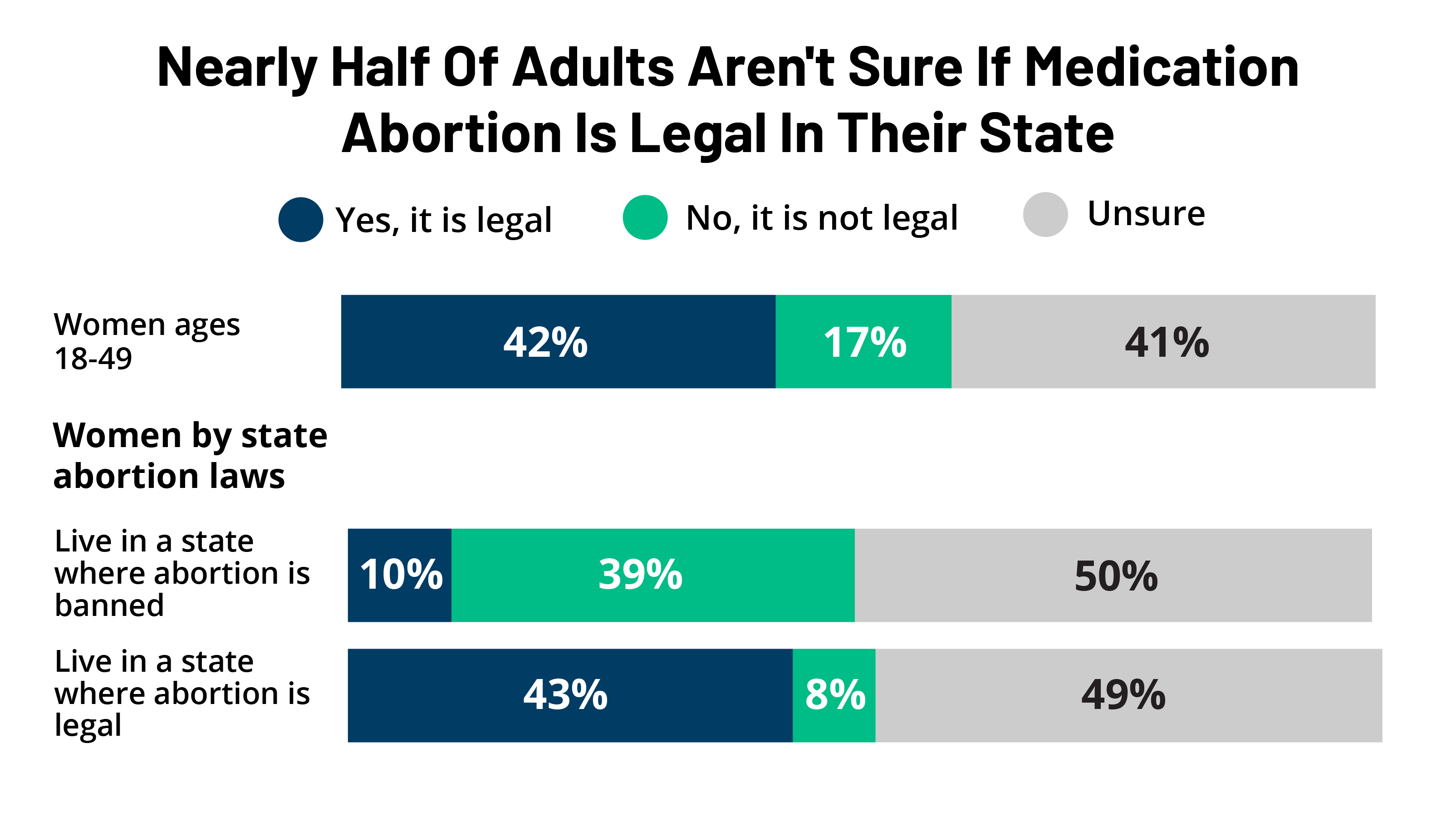
KFF Health Tracking Poll: Early 2023 Update On Public Awareness On Abortion and Emergency Contraception
Pro-life groups slam FDA after Supreme Court mifepristone arguments
- AA Cup Triangle Breast Silicone Breast Forms CD TG Fake Boobs Bra Enhancers

- MYADDICTION Special Pocket Bra with Silicone Breast Form False Boobs Mastectomy AA Cup CC Cup Clothing Shoes & Accessories, Womens Clothing, Intimates & Sleep

- AA Size Cup Soft Underwire Bra for Men. Crossdresser Bra Lingerie

- Silicone Breast Forms,Self-Adhesive Medical Silicone Fake Breast AAA Cup (300G) - KK Cup (6Kg) /Pair for Mastectomy, Breast Enhancement - Reusable,AA Cup : : Fashion

- Beija Spanish Textured Swimwear Fabric Get Wet X Halter Bikini Top in Black

- Shea Whitney - Am I a lucky mama or what?? These boys are just the sweetest & cutest❤️❤️
- 1/2 Dr. Adjustable F/R Air Impact Wrench Max Torque 250ft./lb Air Impact

- CBGELRT Womens Tops Casual Women Spring Tops Womens Corset Top

- Porous Tape 3 Pack Soft Fabric Cloth Breathable Surgical/Medical Adhesive Tap

- The Last Of Us Part II 2 Official Collectors Edition Ellie Statue Figure NO GAME

