physical chemistry - Why do some gases have lower value of Z for a particular pressure? - Chemistry Stack Exchange

By A Mystery Man Writer
In the above graph,the minima of the curve for methane is more than that of nitrogen. Also, for a given value of pressure, the value of $Z$ for methane is less than that of nitrogen. They seem to m
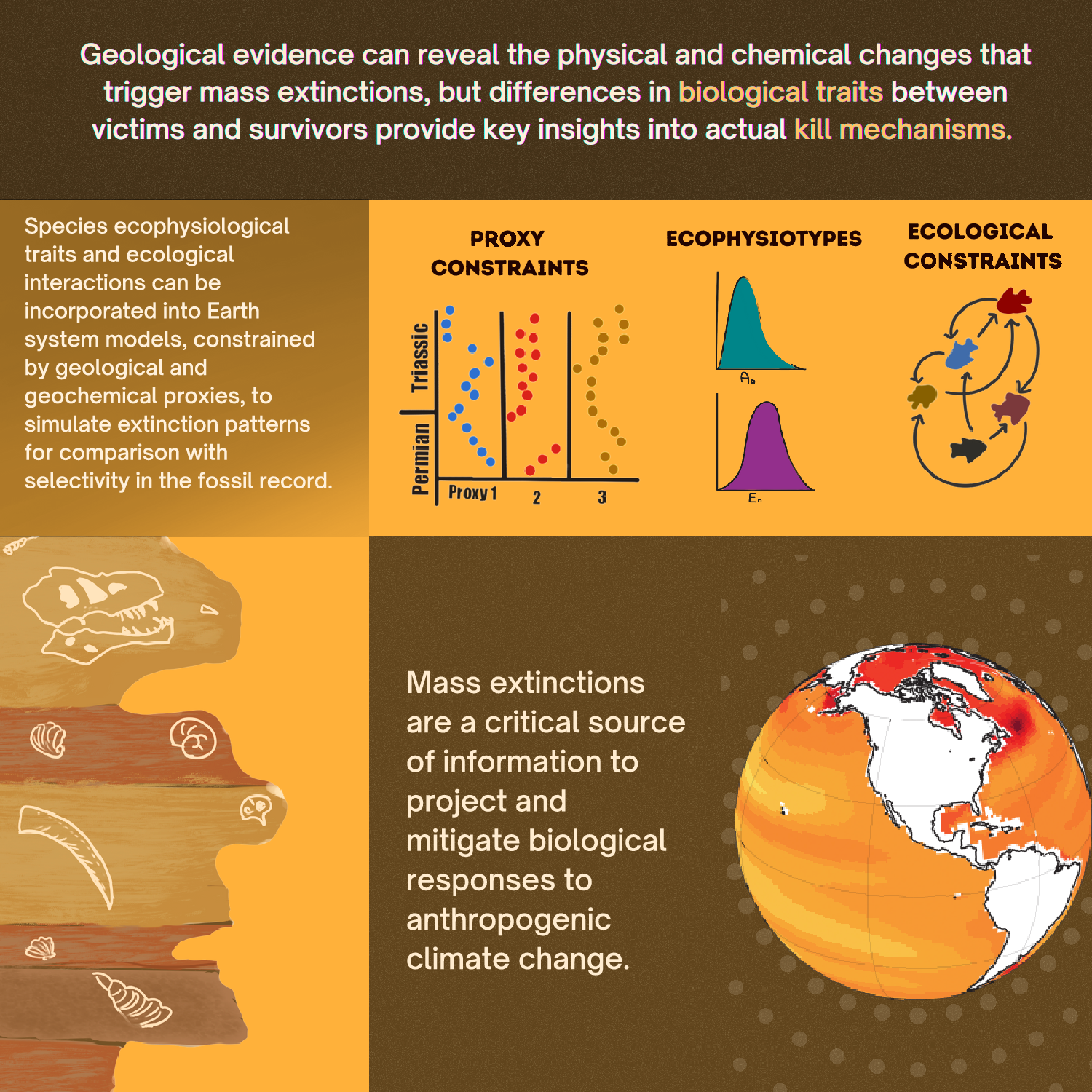
Selectivity of mass extinctions: Patterns, processes, and future
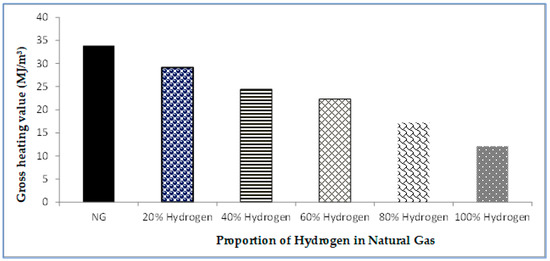
Gases, Free Full-Text

The Conversion of Carbon Monoxide and Carbon Dioxide by
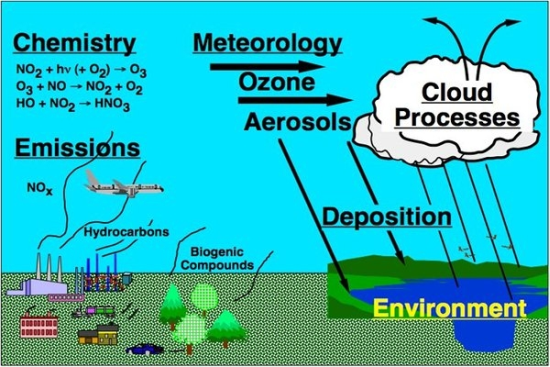
Atmosphere, Free Full-Text

Water Phase Diagram, Comparisons & Importance - Lesson

How Did We Get Here? The Tangled History of the Second Law of
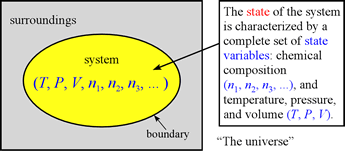
CHEM 101 - Gases and the ideal gas law
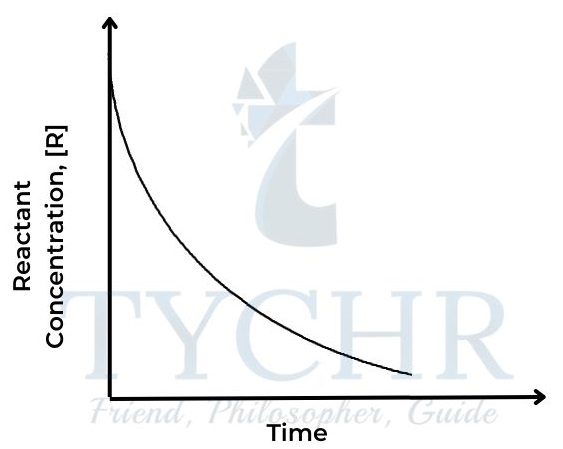
IB Chemistry, Chemical Kinetics Notes
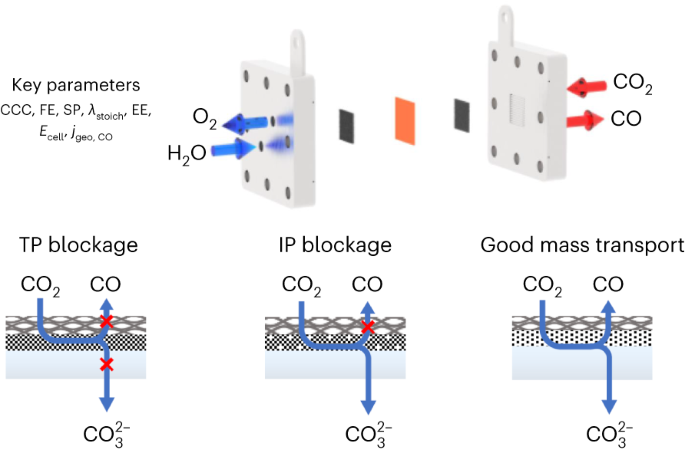
Design and diagnosis of high-performance CO2-to-CO electrolyzer
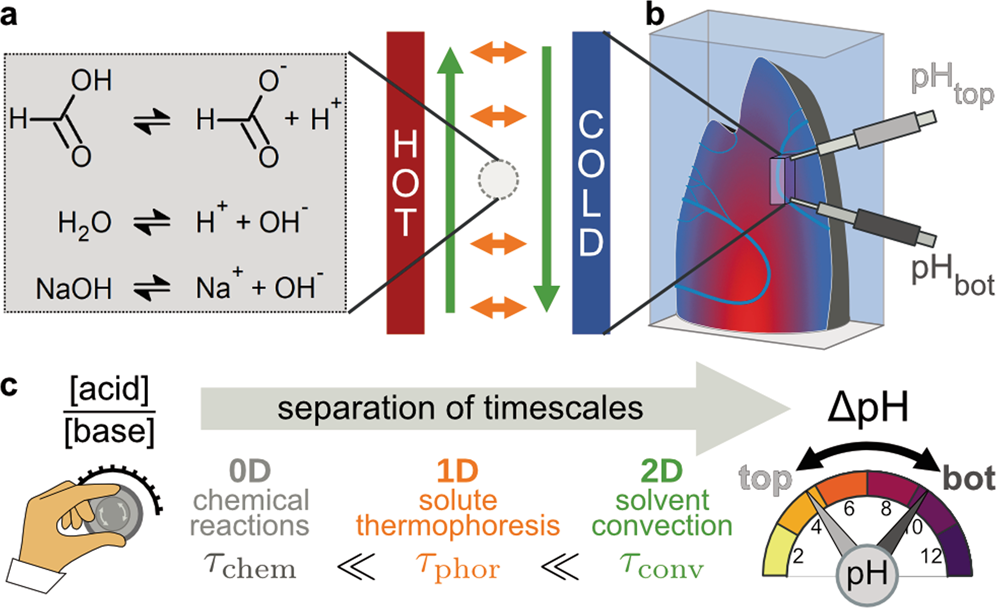
Formation mechanism of thermally controlled pH gradients

General Chemistry/Print version - Wikibooks, open books for an

Partial Pressure- Formula, Dalton's Law, Mixture of Ideal Gas
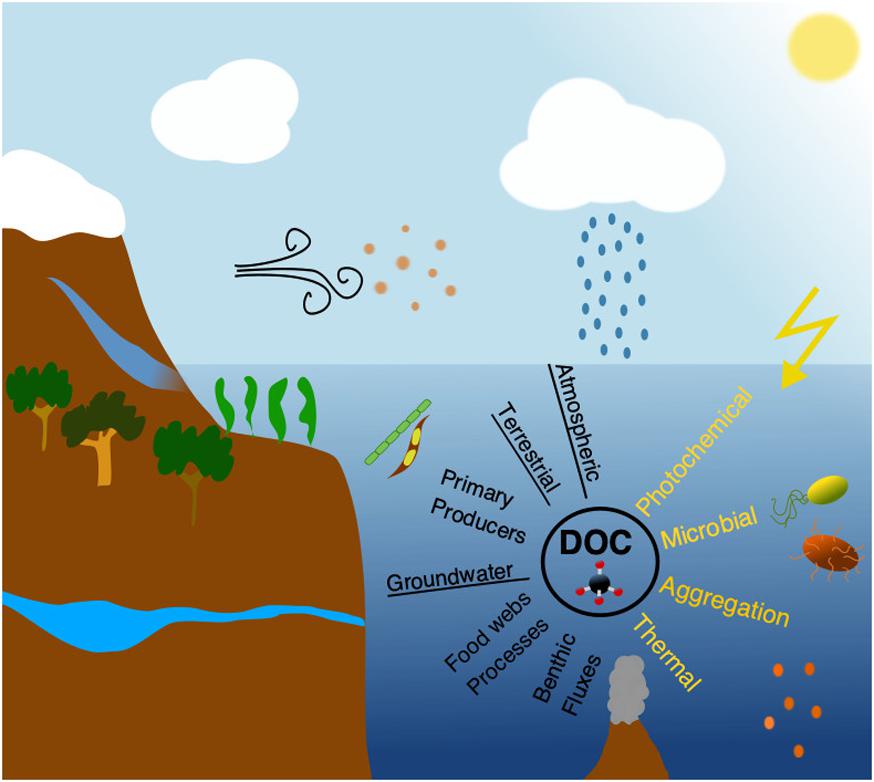
Frontiers Impacts of Global Change on Ocean Dissolved Organic
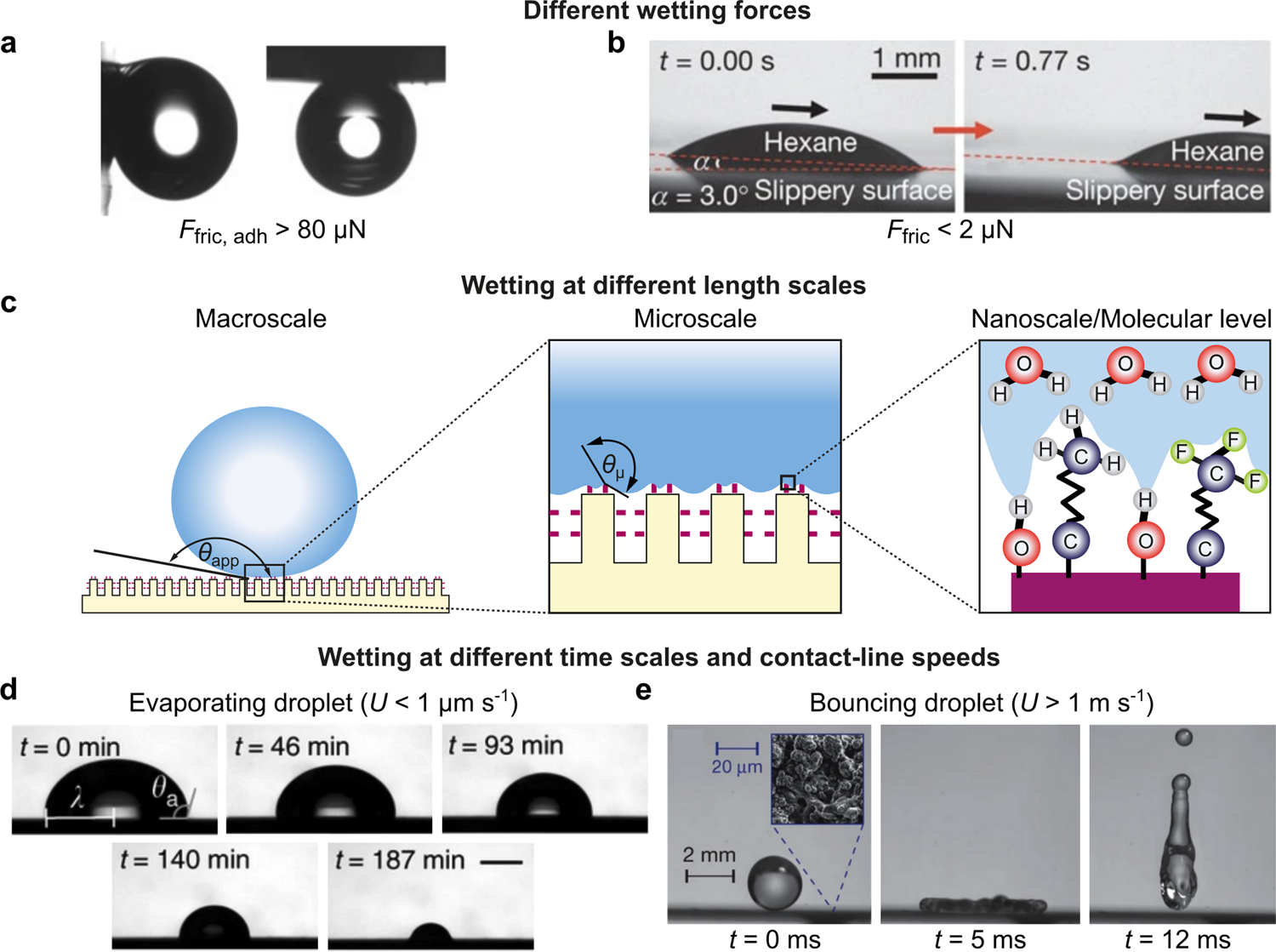
Probing surface wetting across multiple force, length and time
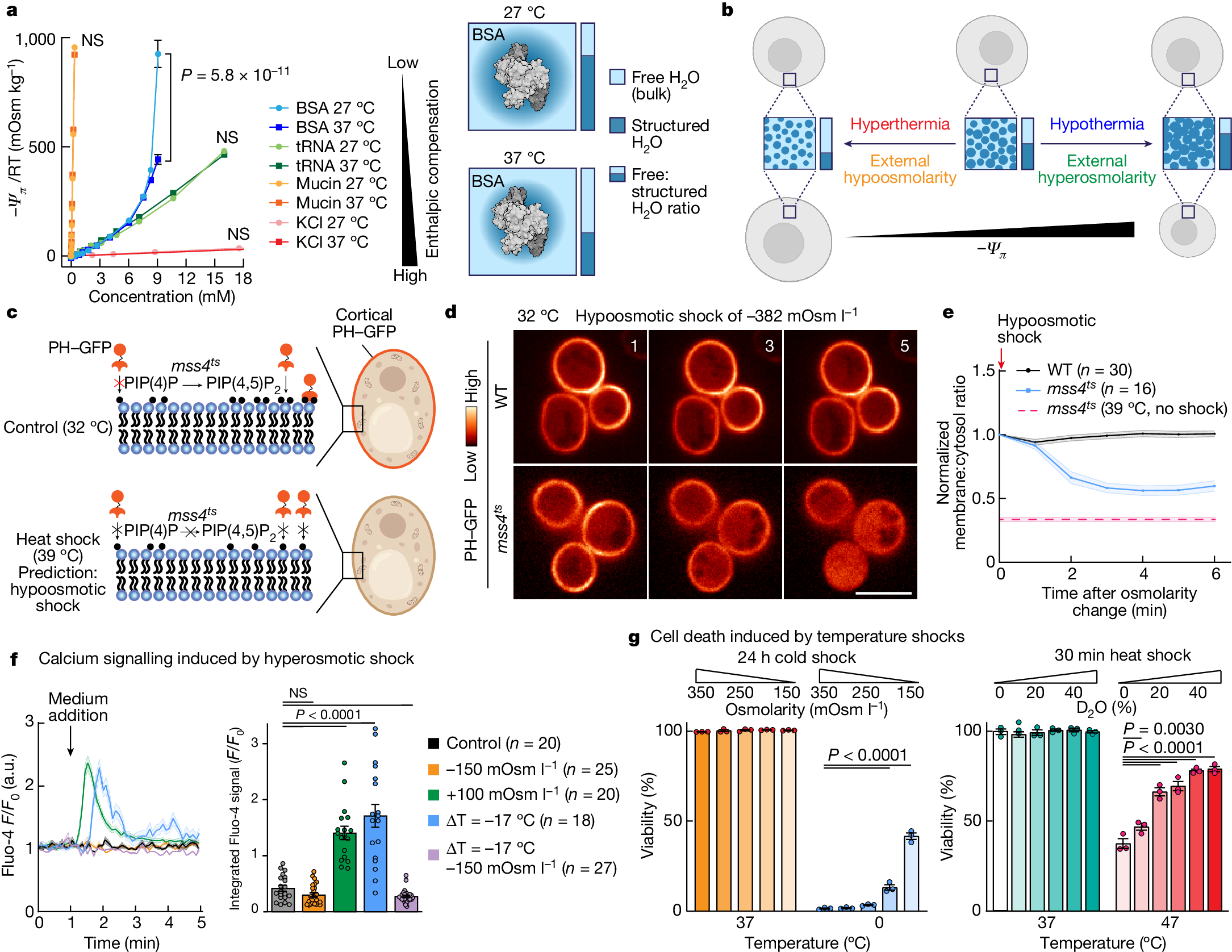
Macromolecular condensation buffers intracellular water potential
- Solved QUESTION 3 Determine the compressibility

- Graph of Compressibility Factor (Z) versus Pressure (Atm

- Compressibility Factor (Z) And Pressure Bar Royalty Free SVG
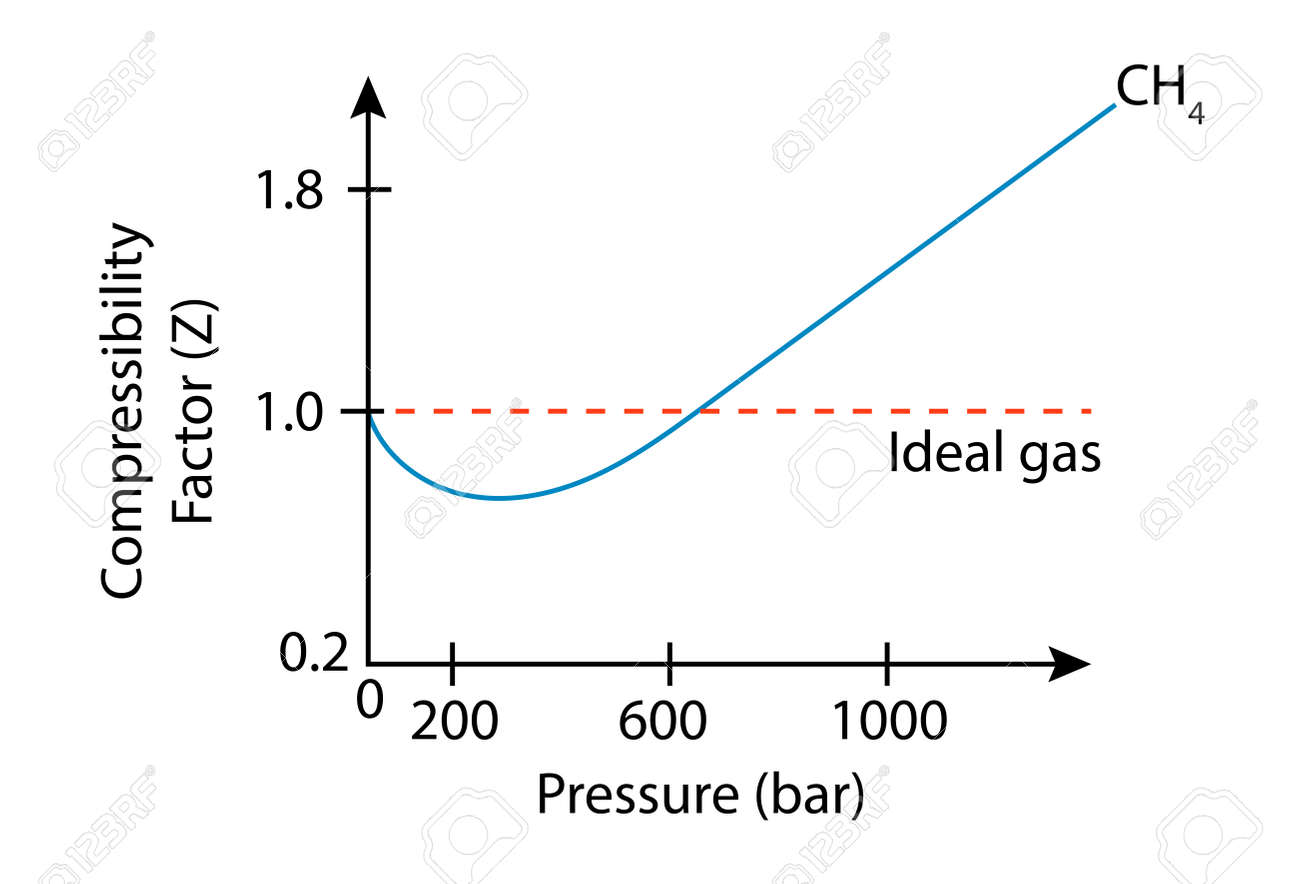
- physical chemistry - Why do some gases have lower value of Z for a

- Compressibility factor Z for sub-critical pressures in a 'one-cell' formula for excel spreadsheets

- Umitay christmas sweatshirt Women's Casual Fashion Christmas Print Long Sleeve O Neck Pullover Top Blouse Sweatshirt

- Plaid Pattern Zip Up Dress, Casual Sleeveless Suspender Dress, Women's Clothing

- CCM PP8 Black Referee Pants

- 6 ferramentas para oficina mecânica que agregam valor ao serviço

- Altheanray Womens Underwear Seamless Cotton Briefs Panties for Women 6 Pack, Hipster Underwear - B/Dg, Large : : Clothing, Shoes & Accessories
